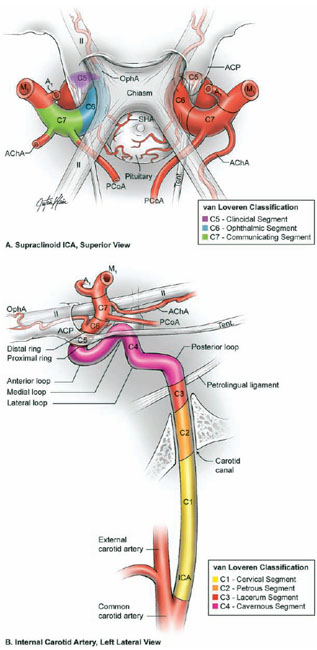
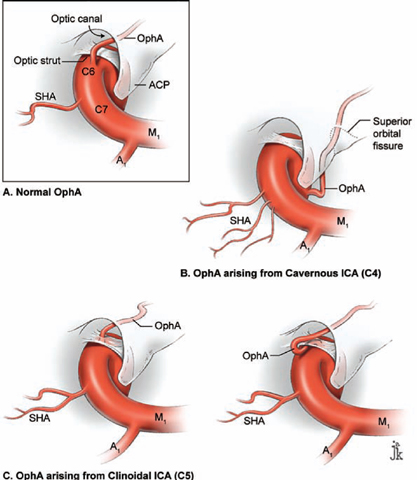
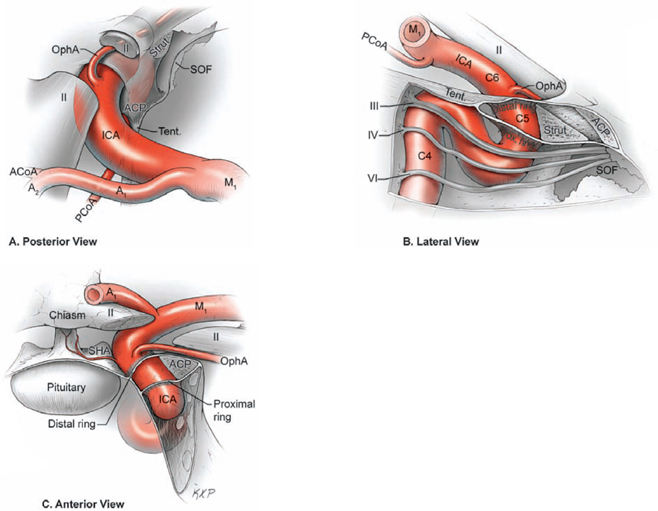
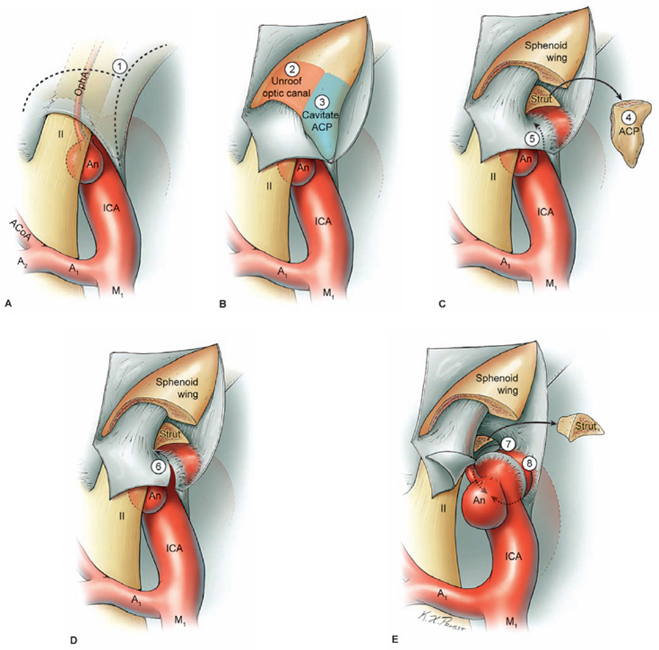
17 Ophthalmic Artery Aneurysms Ophthalmic artery (OphA) aneurysms originate from the ophthalmic segment of the internal carotid artery (ICA). Simple classifications such as Rhoton’s describe only four ICA segments: cervical (C1), petrous (C2), cavernous (C3), and supraclinoid (C4). A contemporary classification by van Loveren and colleagues defines seven segments using the surrounding anatomy and compartments through which the ICA travels, numbered in the direction of antegrade blood flow (Fig. 17.1): cervical (C1), petrous (C2), lacerum (C3), cavernous (C4), clinoidal (C5), ophthalmic (C6), and communicating (C7). The cervical segment begins at the ICA’s origin at the carotid bifurcation and extends to the skull base. The petrous segment begins at the ICA’s entrance into the carotid canal and terminates at its exit from the carotid canal. The lacerum segment runs over, not through, the foramen lacerum for a short distance and ends at the petrolingual ligament. The cavernous segment extends through the cavernous sinus, beginning as it enters at the lateral dural ring and ending as it exits at the proximal dural ring. The clinoidal segment abuts the anterior clinoid process (ACP), extending between the proximal and distal dural rings. The ICA then enters the subarachnoid space and the ophthalmic segment courses from the distal dural ring to the posterior communicating artery (PCoA). The communicating segment runs from the PCoA to the ICA terminus. The clinoidal and ophthalmic segments together are referred to as the paraclinoid ICA because of their intimacy with the ACP. Dolenc defined four right-angle bends along the intracranial ICA. The posterior loop is a turn in the petrous segment as it changes from a vertical, ascending course to a horizontal, medial course. The lateral loop is the second turn, located along the cavernous segment as it returns to a vertical, ascending course. The medial loop is next, also located along the cavernous segment, changing course from ascending to horizontal and anterior. The medial loop is only slightly more medial than the lateral loop, as seen in the frontal view. The anterior loop, also known as the carotid siphon, contains the distal cavernous segment, the entire clinoidal segment, and the proximal ophthalmic segment, as well as the proximal and distal dural rings. Extradural ICA branches are few. The petrous segment gives rise to the caroticotympanic artery and the vidian artery of the pterygoid canal. The cavernous segment gives rise at the medial loop to the meningohypophyseal trunk, which trifurcates into the inferior hypophyseal artery, dorsal meningeal artery, and tentorial artery of Bernasconi and Cassinari. The inferolateral trunk and McConnell’s capsular artery arise more distally on the cavernous segment. None of these extradural arteries is relevant to intradural aneurysms; they are seen with other pathologies such as dural arteriovenous fistulas and meningiomas. Only two named arteries branch from the 1.5-cm length of the ophthalmic segment: the OphA and the superior hypophyseal artery. The OphA originates from the superior surface of the ICA just beyond the distal dural ring. It turns anteriorly under the optic nerve to enter the optic canal and follows the nerve to the orbit (Fig. 17.2A). In 8% of patients, the OphA originates from other spots including the cavernous ICA (Fig. 17.2B), the clinoidal ICA (Fig. 17.2C), and the middle meningeal artery. The superior hypophyseal artery (SHA) originates from the medial surface of the ICA, also beyond the distal dural ring, but the ring’s downward slant along its posterior course positions the SHA origin posterior to the OphA origin (Fig. 17.2). The SHA is approximately 90 degrees medial to the OphA, as seen on the ICA cross section in the frontal view. The SHA courses medially to the sella to supply the pituitary stalk, pituitary gland, optic nerve, chiasm, and floor of the third ventricle. The caliber of the SHA is smaller than that of the OphA, and is even smaller when the SHA is a group of small perforators rather than a single artery. Paraclinoid aneurysms are categorized as ophthalmic, superior hypophyseal, or variant aneurysms (Fig. 17.3). OphA aneurysms arise from the superior (dorsal) surface of the ICA in clear relation to the OphA and distal to its origin. The aneurysm lies at the end of the siphon’s curve and projects superiorly in the direction of blood flow around the bend. The dome impacts the lateral half of the optic nerve because the nerve angles medially along its course to the chiasm and the ICA curves laterally along its supraclinoid course. The optic nerve is typically displaced superiorly and medially, with its superolateral aspect pressed against the falciform ligament and accounting for the inferomedial (lower nasal) quandrantanopsia detected in many patients. Contact between a large aneurysm dome and the optic nerve can be appreciated angiographically as “notching” the aneurysm and “closing” the carotid siphon (the curve tightens as the efferent ophthalmic segment is pushed down toward the afferent cavernous segment, as seen on lateral angiography). The origin of the OphA varies medially to laterally along the superior surface of the ICA and changes the relationship between the dome and the nerve. The OphA with a superomedial origin produces a medially projecting aneurysm that impacts the medial half of the optic nerve and tilts it laterally. Other superiorly projecting OphA aneurysms impact the middle of the nerve and drape it over the dome. The interval between the superior surface of the ICA and the inferior surface of the optic nerve also varies; wide intervals spare the nerve and tight intervals worsen its impingement. Fig. 17.1 Microsurgical anatomy of the internal carotid artery and ophthalmic artery. Superior (A) and left lateral (B) view of the internal carotid artery (ICA) showing its segmental anatomy: C1, cervical segment; C2, petrous segment; C3, lacerum segment; C4, cavernous segment; C5, clinoidal segment; C6, ophthalmic segment; and C7, communicating segment. Dolenc’s loops of cavernous ICA are also shown: anterior loop, medial loop, lateral loop, and posterior loop. AChA, anterior choroidal artery; ACP, anterior clinoid process; OphA, ophthalmic artery; PCoA, posterior communicating artery; SHA, superior hypophyseal artery; Tent, tentorium. Fig. 17.2 (A) The OphA originates from the ophthalmic segment of the ICA (C6) on its superior surface, courses under the optic nerve, and enters the optic canal (superior view, right ICA). The OphA can originate from the cavernous segment (C4) (B) or from the clinoidal segment (C5) (C) of ICA. The SHA originates from the medial surface of the ICA, also beyond the distal dural ring, but posterior to OphA origin and approximately 90 degrees more medial. Superior hypophyseal artery aneurysms arise from the inferomedial surface of the ICA in clear relation to the SHA and with no relation to the OphA (Fig. 17.3). Like OphA aneurysms, SHA aneurysms lie at the end of the carotid siphon, but their formation relates to the lateral curvature of the supraclinoid ICA. These aneurysms project medially toward the sella in the direction of blood flow around this bend. They are distal to the distal dural ring and sit on the diaphragma sella, but larger ones can burrow beneath the diaphragma or forward into the carotid cave, which is an out-pouching of subarachnoid space into the medial clinoidal space where the distal dural ring is thinned or depressed along its medial aspect at the end of the carotid sulcus of the sphenoid bone. SHA aneurysms impact the optic nerve when they are large, and parasellar projection elevates the optic chiasm to produce bitemporal hemianopsia like a pituitary tumor. Growth in this direction “opens” the carotid siphon (the siphon’s curve widens as the efferent ophthalmic segment is lifted away from the afferent cavernous segment, as seen on lateral angiography). Variant aneurysms include dorsal carotid aneurysms, carotid cave aneurysms, clinoidal segment aneurysms, and ventral carotid aneurysms (Fig. 17.3). Dorsal carotid aneurysms are located on the superior wall of ophthalmic segment several millimeters distal to the ophthalmic artery origin and have no relationship to the ophthalmic artery or any other branch. They are small, blister-shaped, and caused by hemodynamic stress or arterial dissection. Carotid cave aneurysms are located in the cave where the distal dural ring thins and invaginates proximally. The aneurysm lies in the subarachnoid space, originates from the medial carotid wall at the end of the carotid sulcus, and has no relationship to other branch arteries. When the ICA is viewed in cross section, the cave aneurysm has a latitude similar to that of the SHA aneurysm but is more proximal. Clinoidal segment aneurysms originate even further proximally, between the dural rings. They originate below the plane of the ACP’s superior surface and proximal to the ophthalmic artery, as seen on lateral angiography. One variant originates from the lateral wall of the clinoidal segment, projects supero laterally, erodes into the ACP and optic strut, and is often associated with an early ophthalmic artery arising from the clinoidal segment (anterolateral variant). The other variant originates from the medial wall of the clinoidal segment, projects superomedially, and remains outside the subarachnoid space when small (medial variant). The medial variant aneurysm arises from an extradural spot just proximal to the carotid cave, and is differentiated from cave aneurysms by its more superior projection. Ventral carotid wall aneurysms originate from the inferior surface of the ophthalmic segment, distal to the distal dural ring. This aneurysm resembles the PCoA aneurysm, but arises proximal, not distal, to the PCoA and has no relationship to a branch artery at this point of origin. Fig. 17.3 The OphA, SHA, and variant aneurysms in the paraclinoid region, as viewed from lateral (A), superior (B), and anterior (C) perspectives. Variant aneurysms include dorsal carotid aneurysms (Dor), carotid cave aneurysms (Cave), clinoidal segment aneurysms (Clin), and ventral carotid aneurysms (Ven). The term clinoid was derived from the Greek word klineios, which means “resembling a bed post.” The ACP is a small triangle of bone with a 1-cm base and a 1-cm height (Fig. 17.4A). It projects posteriorly as the medial continuation of the sphenoid ridge (or lesser wing of the sphenoid bone). It attaches medially to the roof of the optic canal (or anterior root of the lesser sphenoid wing), and is buttressed below by the optic strut (or posterior root of the lesser sphenoid wing). The base of the ACP forms the lateral wall of the optic canal and the superior wall of the superior orbital fissure. The medial aspect of the ACP abuts and is indented by the clinoidal segment of the ICA. The ACP has dense cortical bone along these surfaces and cancellous bone at its core. The optic strut occupies a critical position at the intersection of the optic canal, superior orbital fissure, and orbital apex, and it separates the optic nerve, anterior cavernous sinus, and carotid siphon (Fig. 17.4B). The strut originates from the body of the sphenoid and runs superolaterally to the inferomedial aspect of the ACP. The sphenoid sinus can occasionally extend into the optic strut through the opticocarotid recess and reach the ACP, with ramifications for wound closure after clinoidectomy. The superomedial surface of the optic strut forms the floor of the optic canal, and the inferolateral surface forms the roof of the superior orbital fissure. The strut’s posterior face is the wall against which the anterior loop of the cavernous ICA turns. Fig. 17.4 Microsurgical anatomy of the anterior clinoid process, as viewed from superior (with a section of the optic nerve removed to show the course of OphA) (A), lateral (after an anterior clinoidectomy to show the dural rings as continuations of the dura covering the ACP) (B), and anterior perspectives (with optic strut removed to show its relationship to the carotid siphon and ACP) (C). ACoA, anterior communicating artery. Dura lining the anterior cranial fossa floor, anterior temporal fossa, and sphenoid ridge converge at the ACP. The falciform ligament is the dural edge that runs from the posterior tip of the ACP to the planum sphenoidale, crossing the roof of the optic canal. Dural reflections beneath the falciform ligament fold into the optic canal to form the optic sheath. The ACP is the site of attachment of the anteromedial tentorium and interclinoidal dural fold, two borders of the oculomotor triangle through which CN3 travels before entering its dural sheath en route to the cavernous sinus. Dura arising from the superomedial aspect of the ACP continues medially in an oblique plane that intersects with the ICA to form the distal dural ring (Fig. 17.4C). Dura arising from the inferomedial aspect of the ACP continues medially in a flat axial plane that intersects with the ICA to form the proximal dural ring. The superior dural plane slants downward in the anterior-posterior axis and slants inward in the lateral-medial axis. This layer lies beneath the dura covering the superior aspect of the ACP, and is visible only after reflecting this superficial dura, removing the bone of the medial ACP, and opening the optic sheath. The superior dural plane’s slant intersects with both the ICA and the optic nerve, making it the origin of the distal dural ring and the dura lining the optic canal floor. This dura is thick laterally but thins medially as it courses to the diaphragma sellae and into the carotid cave. The distal dural ring is not a natural anatomic structure; it is a surgical artifact created by a circumferential incision around the ICA as it penetrates this dural divide. The inferior plane is a thin, translucent layer of dura that forms the roof of the cavernous sinus, through which venous blood can be seen. It is called the carotid-oculomotor membrane because it courses from the oculomotor nerve laterally to the ICA medially. Unlike the superior plane that originates from the superomedial edge of the ACP, the inferior dural plane originates from the lateral wall of the cavernous sinus, lines the inferior surface of the ACP, and continues medially past the ACP’s inferomedial edge. Its intersection with the ICA is not as sharp as the superior plane, resulting in a funnel-shaped extension of the cavernous sinus that collars the clinoidal segment. This carotid collar contains venous channels that communicate with the cavernous sinus and can appear as a venous plexus extending as far as the distal dural ring. Like the distal dural ring, the proximal ring is not a natural anatomic structure but a surgical artifact created by a circumferential dural incision around the ICA. Such an incision would open the cavernous sinus and induce brisk venous bleeding. The carotid-oculomotor membrane and dura lining the superior aspect of the ACP fuse into a single layer at the posterior tip of the ACP and the apex of the oculomotor triangle. Thus, the dural rings define important anatomic landmarks. The proximal dural ring marks the termination of the cavernous segment of the ICA and the beginning of the clinoidal segment. The distal dural ring marks the termination of the clinoidal segment and the beginning of its ophthalmic segment. The distal ring is also the barrier between the extra- and intradural compartments, or the beginning of the subarachnoid course of the ICA. An important triangle in the dissection of OphA aneurysms is the clinoidal triangle, which is the space between the optic and oculomotor nerves, with the optic strut at its apex, the dural rings in its midportion, and the roof of the cavernous sinus posteriorly. The standard pterional approach is modified slightly with OphA aneurysms. Head extension that normally allows gravity to retract the frontal lobe also steepens the view into the clinoidal triangle. Therefore, the head is positioned with less extension than with other aneurysms, with the anterior cranial fossa floor oriented vertically. The head is rotated laterally an additional 10 to 20 degrees to improve the lateral view under the optic nerve into the clinoidal triangle. Normally, the pterion is thinned to an egg-shell layer over the orbital roof and lateral wall that preserves the integrity of the orbit. With an anterior clinoidectomy, the posterior orbit is opened around the base of the ACP by up-fracturing the roof at the lateral extent of the superior orbital fissure. Periorbita is stripped from the inside of the orbit and preserved to contain periorbital fat. The superior orbital fissure is widened by rongeuring the lateral plate of bone at the front of the temporal fossa that also forms the intracranial portion of the lateral orbital wall. The dural fold within the superior orbital fissure running between the anterior temporal dura and the periorbita mobilizes laterally, and the posterior orbital roof and the medial end of the sphenoid ridge can be encircled. Rongeuring this bone begins the process of detaching the ACP from its lateral attachment. An anterior clinoidectomy can be completed extradurally by continuing this resection (Dolenc approach), but the intradural resection is preferred because it visualizes the aneurysm and enables immediate clipping if the aneurysm ruptures prematurely. The cervical ICA is routinely prepared and draped whenever the ACP is going to be removed. The decision to open the neck is individualized, and is rare with small, unruptured aneurysms. Neck exposure should be anticipated with ruptured aneurysms and large or giant aneurysms that might require softening or suction decompression. The clinoidal segment offers some proximal control in the cranial field, but the bony canal of the carotid sulcus prevents the tips of a temporary clip from advancing completely across the ICA, and occlusion of aneurysm inflow may be incomplete. Intraoperative angiography is more useful with ophthalmic artery aneurysms than with any other aneurysm because of difficulties in visualizing the aneurysm neck, dissecting around the cavernous sinus, and working beneath the optic nerve. Preparations for intraoperative angiography are made at the start of the procedure by preparing the groin or by inserting a sheath in the femoral artery. Superficial dura on the ACP is incised in an arc that extends from the tip of the ACP posteriorly to the sphenoid ridge laterally, deep to the temporal veins bridging to the spheno-parietal sinus (Fig. 17.5A, step 1). This dural incision extends posteriorly into the oculomotor triangle and down to the oculomotor sheath to completely uncover the posterior tip of ACP. The ACP adheres to the carotid-oculomotor membrane and to superficial clinoidal dura as they converge posteriorly, and attachments of the tentorium and interclinoidal dural fold can cover bony irregularities that fixate the ACP. For example, the middle clinoid process is a small prominence at the medial end of the carotid sulcus that can bridge upwards with the tip of the ACP to form an osseus ring (carotico clinoidal foramen). An osseus bridge can also form between the anterior and posterior clinoid processes. A second dural incision is made from the middle of the first cut, arcing medially across the roof of the optic canal onto the planum sphenoidale to the medial aspect of the falciform ligament. This second incision extends above the rongeured base of the ACP to connect the intradural resection with the extradural resection. Two dural flaps are elevated with round knives, the first one folding posteriorly to protect the optic nerve and aneurysm dome, and the second one folding laterally to expose the ACP’s lateral margin. The optic canal is unroofed with a high-speed drill and a round diamond-tipped drill bit (2 mm diameter) (Fig. 17.5B, step 2). Early decompression of optic nerve not only relieves pressure and distortion from the aneurysm, but also improves the nerve’s tolerance to clinoidal dissection later. Bone forming the optic roof is thinned with the drill, dissected from the optic sheath with a round knife, and upfractured away from the optic nerve. Copious irrigation with cold saline and frequent pauses help dissipate the heat from the drill. The optic canal is unroofed to the medial wall and then 1 cm anteriorly.
 Microsurgical Anatomy
Microsurgical Anatomy
Arterial Anatomy
Aneurysm Anatomy

Anterior Clinoid Process Anatomy
 Aneurysm Dissection Strategy
Aneurysm Dissection Strategy
Modifications in Approach
Removing the Anterior Clinoid Process
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree