Levodopa plasma concentrations with standard oral therapy, for a patient taking five daily doses of levodopa/carbidopa perorally (100+100+75+75+100mg every second hour).
A fast way of establishing whether levodopa has had an effect in the individual patient is to perform a so-called levodopa test. The patient is kept without PD medication for some hours (normally overnight) and then administered a standard peroral dose of levodopa. The effect of this dose on the motor symptomatology is monitored by clinical observation/examination, often including quantitative measurements of movement speed. A positive levodopa test is a good predictor of the effect of long-term dopaminergic treatment and aids in the diagnosis of idiopathic PD [30]. To define the levodopa test as positive, the recommended cut-off value consists of an improvement of the Unified Parkinson’s Disease Rating Scale (UPDRS) III motor score by 14.5% to predict a good effect of long-term dopaminergic treatment or by 18% (to diagnose idiopathic PD) [30]. A negative test must be interpreted with caution, since many patients with a negative test may still respond to long-term dopaminergic therapy. A negative levodopa test should therefore never exclude a trial with dopaminergic treatment.
Sustained-release levodopa preparations
Controlled-release levodopa formulations were developed during the 1980s and became available in 1991. Controlled-release formulations consist of levodopa combined with either carbidopa or benserazide in a matrix designed to delay the release of the active substance. Compared with standard immediate-release tablets, controlled-release formulations have a longer Tmax (time to maximum plasma concentration) and longer t1/2 (biological half-life) [31, 32]. Plasma concentration profiles are less fluctuating and bioavailability is more unpredictable but generally lower than with immediate-release formulations, necessitating a 20–30% higher levodopa dose [33, 34]. Controlled-release formulations have a longer symptomatic effect, but this is counterpoised by a slower onset (Table 7.2).
Pharmacokinetics and pharmacodynamics of levodopa in controlled-release levodopa formulations versus standard-release formulations
| Levodopa pharmacokinetics | |
| Tmax | Longer (2–3-fold) |
| Cmax | Lower (2–3-fold) |
| t1/2 | Unchanged |
| C4-h post-dose | Higher (2-fold) |
| Levodopa pharmacodynamics | |
| Latency to response | Longer |
| Duration of response | Unchanged or shorter |
| Magnitude of response | Unchanged or less |
| Dyskinesias | Unchanged or more |
Tmax, time to peak plasma concentration following dose administration; Cmax, maximum plasma drug concentration after a single-dose administration; t1/2, plasma elimination half-life; C4-h post-dose, concentration 4h after dose administration.
The controlled-release formulations are seldom used in fluctuating patients because of their unpredictable absorption. Indeed, the irregularity of gastric emptying associated with PD becomes even more of a problem with controlled-release formulations compared with standard levodopa tablets, since the former have a lower administration frequency. Controlled-release formulations can be combined with immediate-release formulations to give a faster onset of effect, and this is especially common with the first dose of the day. In some dyskinetic PD patients, the controlled-release formulations have been reported to generate prominent dyskinesias toward the end of the day [33].
Results concerning the efficacy of controlled-release-based treatments versus standard therapy have varied among studies. Two trials have described an improved effect of controlled-release formulations on daily “off” time and/or activities of daily living [35, 36], another trial reported no difference regarding ”on” time without dyskinesias [37], and a long-term study in de novo-treated patients could not demonstrate any advantage of the controlled-release formulations with respect to the incidence of motor fluctuations and dyskinesias [38]. In the clinical practice, controlled-release formulations are seldom used for daytime therapy but are often given as a late evening dose to cover the night hours.
Levodopa and catechol-O-methyltransferase inhibitors
Peripheral inhibition of the metabolism of levodopa via catechol-O-methyltransferase (COMT) prolongs the drug’s elimination half-life and raises its central bioavailability (Table 7.3). This inhibition can be a achieved using the COMT inhibitors entacapone or tolcapone, both in use since the late 1990s (reviewed in [5]). Tolcapone seems to be slightly more effective than entacapone but has a limited use due to the risk of potentially fatal hepatotoxicity. Tolcapone is mostly recommended for patients who have not had a satisfactory effect with entacapone. The use of tolcapone also necessitates regular laboratory liver function tests.
Main effects of catechol-O-methyltransferase inhibitors intake on levodopa pharmacokinetics and pharmacodynamics
| Levodopa pharmacokinetics | |
| Tmax | Unchanged or longer |
| Cmin | Higher |
| Cmax | Unchanged or higher |
| t1/2 | Longer (25–50%) |
| AUC | Larger (40%) |
| Levodopa pharmacodynamics | |
| Latency to response | Unchanged or longer |
| Duration of response | Unchanged or longer |
| Magnitude of response | Unchanged |
| Dyskinesias | Unchanged or stronger |
Tmax, time to peak plasma concentration following dose administration; Cmin/Cmax, minimum/maximum plasma drug concentration after a single-dose administration; t1/2, plasma elimination half-life; AUC, area under the plasma concentration–time curve.
With repeated dosing in clinical routine use, COMT inhibition increases the relative bioavailability and plasma elimination half-life of levodopa. Both Cmin and Cmax are increased, and the degree of fluctuations is relatively unchanged [39, 40]. A slow increase in Cmax during the day has also been reported upon repeated dosing [40]. The clinical benefit of adding a COMT inhibitor therefore mainly consists in decreasing “off” time and increasing “on” time accordingly [41–43]. If the COMT inhibitor is added to an unchanged levodopa dosage in patients with dyskinesias, the latter might increase in severity. Therefore, levodopa should be reduced by 20–30% when adding a COMT inhibitor [41–43].
Since 2003, a combination of levodopa/carbidopa/entacapone in one tablet has been available. The STRIDE-PD study was performed to investigate whether the use of levodopa/carbidopa/entacapone tablets in early-stage untreated patients could reduce the risk of developing dyskinesias [24]. Unexpectedly, initiating treatment with the combined formulation failed to delay or reduce dyskinetic complications of levodopa therapy. In fact, the use of levodopa/carbidopa/entacapone was associated both with a shorter time to onset and with an increased frequency of dyskinesias. These results might depend on the fact that the administration frequency (four doses per day) failed to provide a continuous dopaminergic stimulation and that, moreover, the group treated with the combined formulation received larger levodopa dose equivalents [24].
A new COMT inhibitor in clinical development is opicapone, which provides a very sustained effect making a once-daily administration possible. In a potential clinical application of this treatment, it would be advisable to carefully adjust the levodopa dosage in order to compensate for the relatively enhanced levodopa levels that opicapone will afford [44].
Levodopa and monoamine oxidase type B inhibitors
As an alternative to the approach described above, inhibitors of monoamine oxidase type B (MAO-B, the enzyme that degrades dopamine) can be used to prolong the central effects of levodopa. Two compounds producing a selective and irreversible inactivation of MAO-B are selegiline and rasagiline, both of which are currently used in the treatment of PD (reviewed in [4]). Although there are no direct comparative studies, indirect comparisons would seem to indicate that rasagiline is slightly more effective and better tolerated than selegiline. In a large, double-blinded trial performed in levodopa-treated patients with motor fluctuations, once-daily administration of rasagiline was found to reduce mean daily “off” time and improve PD motor symptoms, an effect similar to that of entacapone [45]. In addition to prolonging the effect of levodopa, MAO-B inhibitors have mild efficacy against PD motor symptoms when used as a monotherapy (reviewed in [4, 5]). In various in vivo and in vitro models of dopamine neuron injury, MAO-B inhibitors exhibited antiapoptotic and antioxidant properties that associated with neuroprotective effects (partly reviewed in [4]). A clinical trial in untreated PD patients has attempted to assess the potential disease-modifying properties of rasagiline using a delayed-start study design. While early treatment with rasagiline at a dose of 1mg/day provided benefits suggestive of a possible disease-modifying effect, early treatment with 2mg/day of rasagiline did not [46]. Because the two very similar drug doses were associated with different outcomes, the results of this study cannot support the hypothesis that rasagiline has disease-modifying properties in PD.
Levodopa and dopamine agonists
Based on their chemical structure, dopamine agonists are divided into two main categories, namely, ergot derivative (e.g. bromocriptine, lisuride, pergolide, cabergoline) and nonergot-type (e.g. ropinirole, pramipexole, rotigotine) compounds. With the exception of apomorphine, all dopamine agonists have a much longer elimination half-life than levodopa and preferential activity at D2/D3 receptors (reviewed in [4]). Accumulating evidence has indicated that some ergot derivatives increase the risk of fibrosis, i.e. an excess formation of connective tissue in organs and body structures (see, for example, the recent statement entitled “Restrictions on use of medicines containing ergot derivatives” issued by the European Medicines Agency at http://www.ema.europa.eu/). Because of this risk, nonergot dopamine agonists are commonly preferred today.
Dopamine agonists are effective as a monotherapy in early disease stages [47, 47]. They are also effective in early combination with levodopa, allowing for a reduction in levodopa dosage. For some of these substances, it has been shown that their late combination with levodopa can improve motor fluctuations [49, 50]. Studies comparing early monotherapy with dopamine agonists versus levodopa have consistently shown than de novo treatment with dopamine agonists gives significantly fewer dyskinesias during a period of 3–5 years [51, 52]. Whether this benefit would persist for a period of longer than 5 years is currently unknown. It is also unknown whether initiating treatment with dopamine agonists will protect from dyskinesia development when levodopa is added later on in the course of PD. Indeed, the addition of levodopa must be taken into consideration at later disease stages, given that dopamine agonist monotherapy may not suffice to manage a more severe parkinsonian syndrome. A number of basic experimental studies have provided indications that dopamine agonists may exert neuroprotective or neurorestorative effects (partly reviewed in [4]), but no clinical study are currently available to support the potential disease-modifying properties of this class of compounds. Dopamine agonists have specific side effects that might limit their clinical usability, including daytime tiredness, impulse control disorders, psychotic symptoms, and heart and lung fibrosis (for ergot-type compounds) (reviewed in [4]).
Levodopa and amantadine
Amantadine was originally developed as a prophylactic treatment against Asian influenza, although it is no longer recommended for this indication. Serendipitous observations in the 1960s indicated that amantadine could reduce parkinsonian symptoms. An early double-blind, placebo-controlled trial of amantadine monotherapy in PD patients concluded that amantadine provided a significant although very mild benefit over placebo, and that this substance could be effective in the long-term treatment of some patients [53]. Today, the most common indication of amantadine in PD is the treatment of levodopa-induced dyskinesias. Indeed, amantadine was the only pharmacological agent to be recognized as efficacious for this indication in a recent evidence-based medicine review [54]. The antidyskinetic and antiparkinsonian properties of amantadine are generally attributed to its antagonistic action at N-methyl-d-aspartate (NMDA) glutamate receptors (reviewed in [4]), although this compound can potentially interact with a number of additional molecular targets.
Novel levodopa formulations in the pipeline
New oral levodopa formulations and prodrugs are currently in different phases of clinical development. A new extended-release formulation of levodopa/carbidopa is IPX066. This product consists of beads, containing levodopa/carbidopa, that dissolve at various rates to allow intestinal absorption to occur over a longer period of time than with standard levodopa/carbidopa tablets. This formulation was compared with standard levodopa/carbidopa in 27 patients in a randomized crossover study over 8+8 days [55]. While plasma levodopa concentrations increased at a similarly rapid rate following a single dose of IPX066 versus standard levodopa, the former resulted in significantly more sustained plasma levels [55]. In a recent study, the drug was compared with immediate-release levodopa in 393 patients with fluctuations, and IPX066 was shown to significantly reduce “off” time [56].
Other treatments under clinical development include a sustained-release levodopa prodrug (XP21279), a carbidopa subcutaneous patch (ND0611), a mixed MAO-B and glutamate inhibitor (safinamide) and inhalable levodopa (reviewed in [57]).
Like IPX066, XP21279 aims to overcome the pharmacokinetic limitations of levodopa and to provide more continuous exposure. However, in contrast to IPX066, XP21279 employs a prodrug that is absorbed by high-capacity nutrient transporters expressed throughout the gastrointestinal tract, which will be enzymatically converted to levodopa inside the body. The engagement of a more widespread transport mechanism is meant to give a larger interface and more time for drug absorption. A phase 2 trial of XP21279 in PD patients with motor fluctuations demonstrated that XP21279 provides less variability in levodopa concentration than the standard oral tablets, and that XP21279-treated subjects had a 30% reduction in mean daily “off” time [58].
ND0611 is a continuously delivered carbidopa solution administered subcutaneously by a pump patch with a number of microneedles [57]. Preclinical studies have suggested that co-administration of ND0611 with standard oral levodopa results in more sustained plasma levels of levodopa. In a phase 1 study in healthy volunteers, ND0611 was administered with levodopa/carbidopa immediate-release preparations, and was found to improve the levodopa half-life, area under the curve, trough levels and time with concentrations above 1000 ng/ml (reviewed in [57]). Further developments of this method consist of combining levodopa and carbidopa in the pump patch. A phase 1, double-blind, placebo-controlled trial in healthy people has shown that this combination is well tolerated and that adequate plasma concentrations are reached (reviewed in [57]).
Fast-acting levodopa formulations can be an aid to the management of motor fluctuations (particularly the “delayed on” phenomenon). An orally administered formulation of levodopa/carbidopa that disintegrates rapidly on the tongue and does not require swallowing is available in some countries (Parcopa®, see http://www.drugs.com/pro/parcopa.html). In addition, effervescent tablets containing the water-soluble levodopa methyl ester melevodopa hydrochloride have been developed recently. It has so far not been possible to demonstrate a significant advantage of these formulations in reducing “off” time above standard levodopa/carbidopa tablets, although trends favoring the fast-acting formulations have been reported by some clinical trials (e.g. [59]). Fast-acting formulations may therefore be useful in certain patients or for particular purposes (e.g. to shorten the interval from drug administration to clinical improvement).
A microtablet formulation containing 5mg of levodopa and 1.25mg of carbidopa is being launched in Sweden. This preparation aims to afford a more individualized levodopa dosage, as 5mg dosing steps can be used. The tablets are delivered by an electronic dose dispenser, making frequent dosing feasible, and also enabling symptom registration by integrating a diary in the portable dispenser. The system has been compared with levodopa/carbidopa/entacapone in a recent phase 1 study. A total of 300mg of levodopa was given to healthy volunteers either as microtablets every 2h and 24 min (75+45+45+45+45+45mg) or as levodopa/carbidopa/entacapone (100/25/200mg) every 6h. The microtablet treatment resulted in significantly more stable levodopa plasma concentrations [60].
Levodopa infusion therapy
The first studies describing intravenous delivery of levodopa were published in 1975 [61] and were soon followed by several other reports showing an improvement of motor fluctuations [62]. In most cases the patients were, however, only treated for a few days. It proved practically difficult to give levodopa intravenously over longer times. The first experiences with intraduodenal levodopa infusion were published in 1986 [63], reporting an effect comparable to intravenous levodopa delivery. This was later confirmed in several other studies (reviewed in [64]). Duodenal delivery methods for broad clinical application have been developed mainly in two different centers, the University of New Brunswick (NB, Canada) and the University of Uppsala (Sweden). A collaboration between the Department of Neurology and the Department of Galenic Pharmacy at the University of Uppsala led to the development of levodopa/carbidopa intestinal gel (LCIG) (reviewed in [65]). This is a combination of levodopa (20mg/ml) and carbidopa (5mg/ml) in the form of a pseudoplastic cellulose-based gel and is delivered with a portable infusion pump. For short-term therapy, a nasoduodenal catheter is used. For long-term treatment, a catheter is inserted into the duodenum by surgical intervention (percutaneous endoscopic gastrostomy or jejunostomy, the latter being performed in only a few cases) (Figure 7.2). Open and randomized controlled studies have shown that LCIG achieves stabilization of both plasma levodopa concentrations and clinical status, producing a strong reduction in motor fluctuations and an overall increase in time spent in the “on” phase (reviewed in [65]). A blinded, randomized, crossover study comparing LCIG monotherapy with individually optimized peroral therapy has demonstrated an increase in daily “on” time from 81 to 100% and an improvement in health-related quality of life upon treatment with LCIG (reviewed in [65]). In a second randomized, double-blind, double-dummy study, 71 patients were randomized to active pump or active levodopa/carbidopa standard therapy [66]. The use of LCIG reduced “off” time and increased “on” time without dyskinesias significantly more than tablet treatment. Health-related quality of life improved significantly more in the LCIG group. A large, open-label study of 192 patients receiving LCIG treatment over 1 year demonstrate improvements in “off” time, “on” time without dyskinesias and health-related quality of life similar to those reported in the randomized controlled study [67]. Preliminary data indicate that moving from peroral levodopa to infusion therapy can also improve nonmotor symptomatology [68].
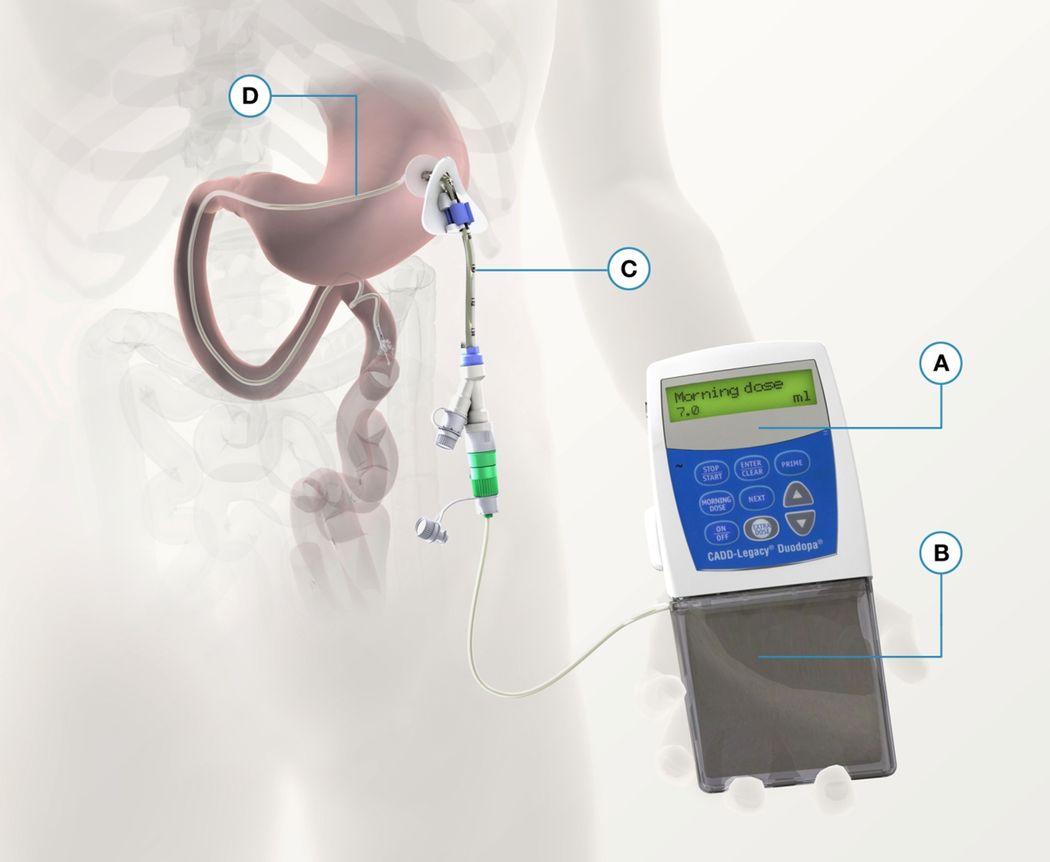
The levodopa/carbidopa gel (LCIG) infusion system. (A) Pump. (B) LCIG cassette. (C) Percutaneous endoscopic gastrostomy. (D) Intestinal tube.
Treatment with LCIG is initially given only during the daytime. In patients experiencing nighttime problems with parkinsonism symptoms and suboptimal sleep, a 24h treatment can bring significant improvement without inducing further side effects or tolerance [69]. Adverse events of LCIG therapy are related to the infusion method. These include peritonitis, dislocation or occlusion of the duodenal catheter, and leakage in the infusion system. Acute or chronic neuropathies and reversible encephalopathies have been reported in some patients receiving LCIG treatment, and a link has been suggested between these conditions and adverse metabolic effects of the treatment, such as deficiency of vitamin B6 or B12 and/or elevated plasma homocysteine levels (reviewed in [5]). However, the pathogenic mechanisms underlying the observed neuropathies/encephalopathies remain to be clarified. The main drawback associated with levodopa infusion therapy does not lie in these infrequent potential complications but rather in its high costs, which would become prohibitive for the healthcare systems in most countries if this treatment were to be prescribed routinely to PD patients with motor fluctuations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



