CHAPTER 31 ORGANIZATION: PYRAMIDAL AND EXTRAPYRAMIDAL SYSTEM
The large brains of primates have afforded remarkable behavioral adaptability. In humans, this adaptability is manifested in many ways, including the capacity for movements generated volitionally or somewhat automatically. Such movements include those required for speech and communication, for independent hand use, and for locomotion. Furthermore, these movements can be engaged in simultaneously, a feat that requires extraordinary adaptive control of the motoneuronal outputs to muscles. This occurs, for example, when a person talks and uses a mobile phone when walking or bicycling. This performance of skilled and complex movements can be generated by all components of the body’s musculature.
Motoneuronal output to generate movements under volitional control is achieved by descending neural drive from so-called motor cortical centers that generate corticospinal and corticobulbar outputs, as well as corticoreticulospinal outputs.1 Since work in the late 19th and early 20th centuries on stimulation of motor cortex to elicit movements and on sectioning of the pyramidal tract to impair them, the important role of motor cortical outputs with axons in the medullary pyramids has been recognized. This emphasis has fitted with observations of paralysis and weaknesses after development of lesions of this system and with evidence for evolution of the size and terminations of the corticospinal system in primates. There are corticospinal outputs not only to interneurons within the spinal cord but also directly to motoneurons (termed corticomotoneuronal connections). The pyramidal tract itself, so named from the pyramidal decussation in the medulla, contains the bulk of direct corticofugal outputs destined to recruit spinal motoneurons.1
A primary role for the motor cortex in movement control has been paralleled by recognition that subcortical nuclei are also critically involved. Two subcortical systems appear crucial for adequate volitional movement: cortical interactions with the motor thalamus and the integration of information through the basal ganglia.2 The basal ganglia comprise a large number of integrated regions (see later discussion) that affect the motor system at the level of the thalamus and brainstem. Just as lesion and stimulation studies show the importance of motor cortical outputs, comparable approaches to study of the basal ganglia and thalamus have revealed that they profoundly gate and modify movements. Depending on the location of lesions or stimulation, there can be extreme poverty of all movements, abnormal postures, and uncontrollable rhythmic motor outputs.3
THE EXTRAPYRAMIDAL MOTOR SYSTEMS
The basal ganglia and thalamus comprise a number of anatomically integrated regions involved in motor control.4,5 They also play important roles in emotional, motivational, associative, and cognitive functions. Basal ganglia regions include the caudate nucleus, putamen, internal and external segments of the globus pallidus, subthalamic nucleus, and substantia nigra. These regions have complex anatomical interconnections that are not fully characterized electrophysiologically. Thalamic regions participating in motor control include specific ventral, posterior, and intralaminar nuclei.6 The understanding of how these regions integrate motor information is based largely on knowledge of their relationship to the corticospinal pyramidal system. There are three types of subcortical extrapyramidal regions to consider: those that receive direct information from pyramidal or cortical regions with major influences on pyramidal regions; those with projections to pyramidal or directly related regions; and those that perform internal monitoring and regulation of extrapyramidal regions.
Subcortical Regions Receiving Pyramidal Input and Their Influence on the Pyramidal System
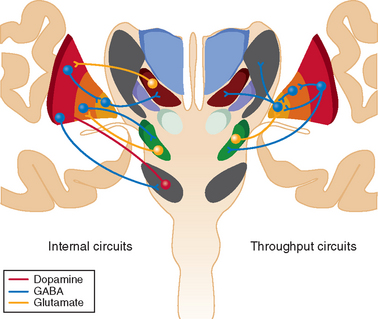
The largest subcortical extrapyramidal region receiving pyramidal input from both supragranular and infragranular regions of the motor cortices is the putamen (Figs. 31-1 and 31-2).4 Together with the caudate nucleus, this striatal area receives an information from all cortical regions. The putamen passes on processed information through both a direct activating and an indirect inactivating pathway to the basal ganglia output nuclei, the internal globus pallidus, and the substantia nigra pars reticulata.4 These basal ganglia regions are small in comparison with the striatum (100-fold fewer neurons) and contain large nonspiny γ-amino butyric acid–ergic (GABAergic) inhibitory projection neurons.
Striatal neurons also receive excitatory thalamic input from the ventral anterior, ventrolateral nuclei, and caudal intralaminar nuclei; receive inhibitory input from the external globus pallidus; and are modulated by dopamine from the substantia nigra pars compacta and mesencephalic tegmentum.4,7 Overall dopamine enhances activity in the direct pathway and decreases activity in the indirect pathway.8 The striatum con tains mainly GABAergic spiny projection neurons and a small population of GABAergic and cholinergic interneurons (≈3% of striatal neurons). Striatal spiny neurons project to the globus pallidus and substantia nigra, as well as giving rise to dense local arbors that contact other spiny neurons.4 They are usually silent and discharge only when cortical information is received. The GABAergic interneurons establish contacts with the dendritic shafts of neighboring spiny neurons and form the structural basis for feedforward striatal surround inhibition. Striatal cholinergic interneurons, in contrast, are tonically active and play a major role in the learning of reward behavior.
The smallest subcortical extrapyramidal region receiving pyramidal input from layer V neurons of the motor cortices is the subthalamic nucleus.9 This is the only cortical input to this nucleus, and this hyperdirect pathway conveys powerful excitatory effects from the motor cortices to the globus pallidus and substantia nigra pars reticulata, bypassing the striatum, with shorter conduction times than in the direct striatal pathway.9 It also receives significant inhibitory input from the globus pallidus and striatum.10 The subthalamic nucleus contains nonspiny excitatory glutamatergic neurons that conduct short-latency excitatory responses to the globus pallidus and substantia nigra after cortical excitation.
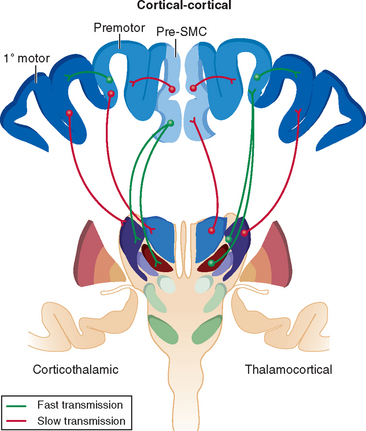
Specific regions of the thalamus also receive pyramidal input from motor cortices.5 There is a reciprocal excitatory component from small layer VI neurons to the ventrolateral thalamus and a fast-conducting nonreciprocal excitatory component from layer V neurons that allows the synchronization of thalamocortical oscillations and information flow across functionally related cortical fields. Cortical regions associated with executive function (such as dorsolateral prefrontal cortex) have nonreciprocal connections to thalamic regions that project to premotor cortices (ventral anterior thalamus), whereas premotor cortices have nonreciprocal connections with thalamic regions that project to the primary motor cortex (ventrolateral thalamus).11 The thalamus contains nonspiny excitatory glutamatergic neurons that conduct short-latency excitatory responses after appropriate cortical excitation. These interconnections between the cortex and thalamus largely determine cortical activity, facilitating information transfer from one cortical region to another through a feedforward mechanism. The output nuclei of the basal ganglia inhibit thalamocortical activity in the ventral anterior, anterior ventrolateral, and caudal intralaminar nuclei.11
Subcortical Regions Participating in the Internal Regulation of Extrapyramidal Systems
There is significant internal modulation of the hyperdirect, direct, and indirect basal ganglia pathways (see Fig. 31-1). One of the basal ganglia regions that most influence striatal processing is the dopaminergic substantia nigra pars compacta, as discussed previously, although pallidal and thalamic projections also significantly modify striatal output. The large, tonically active, nonspiny dopaminergic neurons of the substantia nigra pars compacta receive input directly from striatal spiny projection neurons, which modifies their firing rate and patterns.8 This reinforces wanted behaviors and suppresses unwanted behaviors. These dopaminergic neurons also receive significant innervation from cortical, limbic, and brainstem regions and play a role in shifting attentional sets.
The basal ganglia region with the most internal connections is the external segment of the globus pallidus.4 These pallidal neurons receive most input from the striatum and the subthalamic nucleus, as well as a small dopaminergic projection from the substantia nigra. Individual neurons in the external globus pallidus innervate the output nuclei, the subthalamic nucleus, and the substantia nigra pars compacta.4 About 25% of them also innervate the striatal GABAergic spiny neurons. These neurons provide the anatomical substrate for the synaptic integration of functionally diverse cortical information within the basal ganglia and appear to work in parallel with the subthalamic nucleus and striatum to set up appropriate oscillatory activity within the basal ganglia.10 They are therefore in a position to provide level-setting control of the activity through virtually the whole of the extrapyramidal basal ganglia system.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree