CHAPTER 28 ORTHOSTATIC HYPOTENSION
Maintenance of upright posture is made possible by rapid cardiovascular adaptation that ensures blood flow to vital organs, including the brain, and depends primarily on an intact autonomic nervous system. When this system fails, as occurs in neurological disorders affecting autonomic neurons, orthostatic hypotension results. Severely affected patients are completely disabled, unable to stand for more than few seconds before syncope ensues.
PATHOPHYSIOLOGY
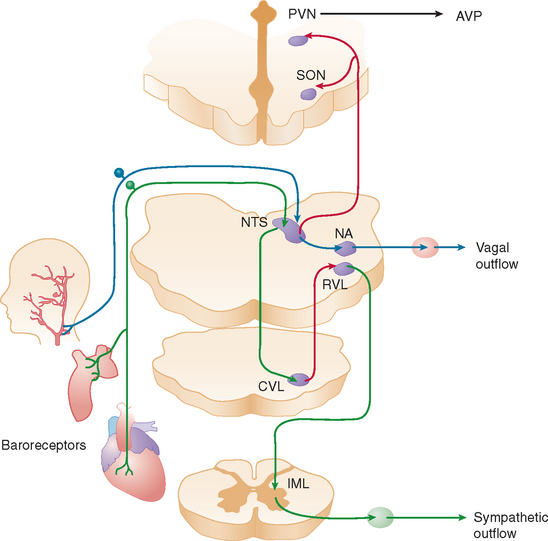
When a normal individual stands, about 700 mL of blood pools in the legs and lower abdominal veins. Venous return decreases, resulting in a transient decline in cardiac output. The reduction in central blood volume and arterial pressure is sensed by pressure-sensitive cardiopulmonary volume receptors and arterial baroreceptors. This leads to baroreflex-mediated sympathetic activation with increases in stroke volume and peripheral vasoconstriction and parasympathetic withdrawal with increase in heart rate (Fig. 28-1). These rapid hemodynamic changes prevent blood pressure from falling; their failure causes orthostatic hypotension.
When arterial blood pressure falls below a critical level, cerebral blood flow also decreases. When systolic blood pressure is around 50 mm Hg at brain level (which corresponds to a systolic blood pressure of approximately 70 mm Hg at cardiac level when a person is standing) (Fig. 28-2), the autoregulatory capacity of cerebral blood flow reaches maximum vasodilation and is unable to compensate for a further fall in blood pressure.
In addition to these almost instantaneous changes in vascular tone and heart rate directly mediated by autonomic innervation, other longer term mechanisms that contribute to the maintenance of upright blood pressure are also influenced by the baroreflex. These mechanisms are impaired in patients with autonomic failure and contribute to their orthostatic hypotension. Increased sympathetic renal nerve activity induces tubular sodium reabsorption directly1 and by stimulating the secretion of renin from the juxtaglomerular apparatus.2 Renin and converting enzyme convert circulating angiotensinogen into angiotensin II, which is a vasoconstrictor and secretagogue of aldosterone from the adrenal medulla. Aldosterone retains sodium in the kidney, increasing extracellular fluid volume. Patients with autonomic failure have low renin and low aldosterone levels. In addition, unloading of thoracic baroreceptors releases the antidiuretic hormone from the neurohypophysis into the systemic circulation.3,4 Acting on specific receptors in vascular smooth muscle cells,5 vasopressin produces vasoconstriction, and in the kidney it causes water retention and expands extracellular fluid volume.6 Baroreflex-mediated vasopressin release is blunted in some patients with autonomic failure.
Two other circulating vasoactive peptides, atrial natriuretic factor (ANF) and endothelin, are involved in the regulation of blood pressure and extracellular fluid volume, and their secretion may also be controlled, at least in part, by autonomic reflexes. ANF is secreted from atrial myocites7 when atrial pressure increases. ANF produces natriuresis, relaxation of vascular smooth muscle, and inhibition of renin and aldosterone secretions.8–10 When right atrial pressure decreases, as during the upright posture, circulating levels of ANF quickly fall, contributing to vasoconstriction and expansion of extracellular fluid volume. Whether ANF is released by the direct effect of pressure on the cardiocytes or by a centrally mediated autonomic reflex is still unclear. Kaufmann and colleagues found that the response of circulating ANF to changes in atrial pressure is preserved in patients with baroreflex impairment, which suggests that a local intracardiac reflex regulates the secretion of the peptide.11 Endothelin, a powerful vasoconstrictor synthesized by endothelial cells,12 has an important role in the local control of the circulation.13 In addition, however, endothelin is synthesized by neurons in the paraventricular and supraoptic nuclei of the hypothalamus14 and is co-released with vasopressin from the neurohypophysis into the bloodstream when thoracic baroreceptors are unloaded.15 The physiological function of the endothelin released into plasma during baroreflex activation remains to be defined, but it is likely that circulating endothelin contributes to the vasoconstriction that maintains blood pressure in the upright posture.15
DIAGNOSIS AND EVALUATION OF ORTHOSTATIC HYPOTENSION
Orthostatic hypotension produces a characteristic clinical history. Symptoms of lightheadedness and tunnel vision occur on standing, never while the person is lying down, and are always relieved immediately on sitting or lying down, because cerebral blood flow is passively restored. Failure to meet these criteria suggests other causes of impaired consciousness (e.g., hypoglycemia, seizures, cardiac arrhythmias).16 In patients with diabetes or other peripheral neuropathies, proprioceptive abnormalities lead to feeling of unsteadiness that patients refer as dizziness, which may wrongly suggest orthostatic hypotension. In addition to the classic lightheadedness with blurred vision and syncope, symptoms of chronic orthostatic hypotension may include vague generalized weakness, fatigue, cognitive impairment, and pain in the shoulders and back of the neck (coat hanger pain).
Many patients with chronic autonomic failure can tolerate very low orthostatic blood pressures with few symptoms of cerebral hypoperfusion, perhaps because their cerebral autoregulatory capacity is well preserved.17 However, physical exercise, prolonged standing, the postprandial state, or mild volume depletion exacerbates orthostatic hypotension and invariably triggers symptoms of cerebral hypoperfusion, including presyncope or syncope.
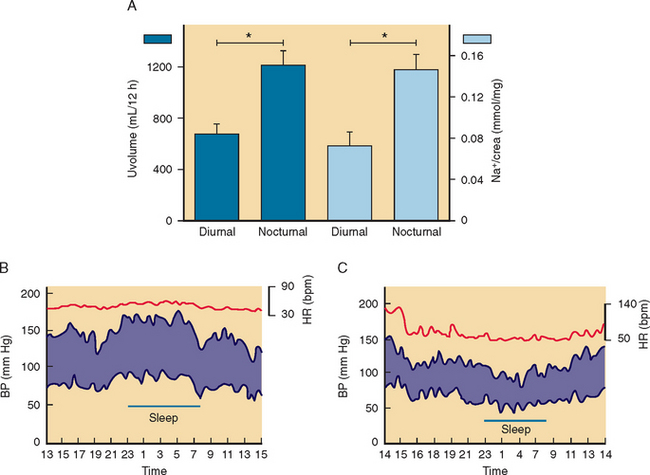
The diagnosis of orthostatic hypotension relies on simple measurements of blood pressure and heart rate after the patient has lain down for 5 to 10 minutes and after 1 to 3 minutes of standing. Orthostatic hypotension is arbitrarily defined as a decrease in systolic blood pressure of at least 20 mm Hg and in diastolic blood pressure of at least 10 mm Hg within 3 minutes of standing, in association with symptoms of cerebral hypoperfusion.18 On occasion, the diagnosis of orthostatic hypotension may require repeated measurements of blood pressure throughout the day. In patients with autonomic impairment, the severity of orthostatic hypotension is worse early in the morning or about 30 minutes after a meal compared to the rest of the day.
Concomitant measurements of heart rate are crucial for adequate interpretation of results. Side effects of medications are arguably the most common causes of orthostatic hypotension. The most common culprits are tricyclic antidepressants, diuretics, nitrates, and α blockers used to treat benign prostatic hypertrophy. In these cases, there is usually a compensatory increase in heart rate in association with orthostatic hypotension. In contrast, the presence of symptomatic orthostatic hypotension without an adequate compensatory increase in heart rate is sufficient clinical indication of autonomic failure (Fig. 28-3). The diagnosis can be easily confirmed with simple measurements of heart rate and blood pressure (Table 28-1). No single test completely differentiates patients with autonomic failure from age-matched control subjects, but together these tests provide a reliable indicator of the presence and severity of cardiovascular autonomic impairment.
TABLE 28-1 Assessment of Autonomic Function: Bedside Physiological Tests