FIGURE 31.1 The light touch, pressure, position, and vibration pathways from the body and face are indicated by the dashed line; the pain and temperature fibers from the body and face are indicated by the solid line. Fibers from these various sources ultimately converge on the ventral posterior nuclei of the thalamus, which projects via the thalamic radiations to the primary sensory cortex in the postcentral gyrus. V trigeminal; VPL, ventral posterior lateral; VPM, ventral posterior medial.
SENSORY RECEPTORS
The interface between the sensory nervous system and the environment is the receptor. There are many different types of receptors in the skin, subcutaneous tissues, muscles, tendons, periosteum, and visceral structures to subserve the transduction of various types of sensory information into nerve impulses. Sensory end organs are found in the skin and mucous membranes throughout the body. They are denser on the tongue, lips, genitalia, and fingertips and farther apart on the upper arms, buttocks, and trunk. One nerve fiber may innervate more than one receptor, and each end organ may receive filaments from more than one nerve fiber. Receptors may respond to more than one type of stimulus but have “specificity” because their threshold is lowest for a particular type of stimulus. Receptor stimulation causes a change in the permeability of its membrane that gives rise to a receptor or generator potential—a local, nonpropagated potential whose intensity is proportional to the intensity of the stimulus. Receptors may adapt to a stimulus to varying degrees. Some receptors are rapidly adapting and most sensitive to on-and-off stimuli. Others adapt slowly and function to constantly monitor a stimulus. Receptors are the terminal part of, and are continuous with, a sensory nerve. Receptor potentials induce action potentials in the nerve, with the frequency of the action potential discharge usually in proportion to the amplitude of the receptor potential, which is in turn proportional to the intensity of the applied stimulus. Each neuron has a specific receptive field, which consists of all the receptors it can respond to. The receptive fields form more or less discrete maps in the nervous system in which specific regions of the body are represented in specific regions of the brain. Some systems have a highly organized map (e.g., the somatosensory homunculus in the postcentral gyrus). In other systems, the maps are crude. In the cortex, neurons subserving the same modality and with similar receptive fields are organized into vertical rows, which extend from the cortical surface to the white matter and are referred to as cortical columns.
Receptors may be free nerve endings (FNE), or they may be encapsulated or connected to specialized nonneural components to form the sense organ. The nonneural elements are not excitable, but they help to form a structure that efficiently stimulates and excites the sensory nerve fiber. Exteroceptors respond to external stimuli and lie at or near the interfaces between the body and the environment. Special sensory exteroceptors subserve vision, hearing, smell, taste, and vestibular function. General or cutaneous sensory organs include the free and encapsulated receptor terminals in the skin. Proprioceptors respond to stimulation of deeper tissues, such as muscles and tendons, and are designed particularly to detect movement and the position of body parts. Receptors around hair follicles are activated by distortion of the hairs.
Receptors may be classified by the specific modality to which they are more responsive, such as mechanoreceptors, thermoreceptors, chemoreceptors, photoreceptors, and osmoreceptors. Mechanoreceptors respond to deformation, such as touch or pressure. Stimulation of mechanoreceptors causes a physical deformation of the receptor that results in the opening of ion channels. Polymodal receptors respond efficiently to more than one modality, especially stimuli that cause tissue damage and pain. There is a great deal of variation in the density of sensory receptors between different body surface regions. Also, receptor density decreases with advancing age.
Receptors may also be classified morphologically, but the correlation between function and morphology is not nearly as close as was once believed. There are FNE, epidermal endings, and encapsulated endings. The FNE are fine, unmyelinated terminal fibers that arborize in the skin, fascia, ligaments, tendons, and other connective tissues throughout the body. They mediate several sensory modalities; some are exclusively nociceptors. The FNE are the terminals of sensory C fibers or A-delta fibers (see “Nerve Fiber Classification”) and are located in both glabrous and hairy skin. The FNE terminals of unmyelinated nerve fibers are mainly nociceptive, but they may also be thermoreceptors or mechanoreceptors. Merkel cell endings (tactile discs or menisci) are specialized nerve endings lying just below the epidermis, especially in glabrous skin, and around hair follicles that function as mechanoreceptors. In encapsulated nerve endings, nonneural cells form a capsule around the terminal axon. Examples include Golgi tendon organs, muscle spindles, Ruffini endings, peritrichial endings, and Meissner’s and Pacinian corpuscles.
There is evidence that abnormalities may be limited to sensory receptors in some neuropathies previously thought to selectively affect small nerve fibers.
NERVE FIBER CLASSIFICATION
In the peripheral nervous system, axons are divided into three major size groups: large myelinated, small myelinated, and unmyelinated. The largest fibers are spindle afferents and motor fibers arising from alpha motor neurons. The smallest, unmyelinated fibers are pain and postganglionic autonomic fibers. Large myelinated axons have diameters in the 6 to 12 mm range, small myelinated axons 2 to 6 mm, and unmyelinated axons 0.2 to 2 mm. Small myelinated fibers are about three times more numerous than large myelinated axons. The conduction velocity (CV) of a fiber depends on its diameter and degree of myelination. Large fibers conduct more rapidly than small ones, and myelinated fibers, more rapidly than unmyelinated ones. The CV ranges from less than 1 m/s for small, unmyelinated fibers to greater than 100 m/s for large, myelinated fibers. In large, myelinated fibers, the fiber diameter (in mm) × 6 approximates the CV (in m/s).
Peripheral nerve fibers are classified by size and CV according to two schemes: the ABC and the I/II/III/IV systems (see Chapter 23). The ABC scheme includes both motor and sensory fibers. The A-alpha and A-gamma fibers are motor. The A-alpha group also includes afferents from encapsulated receptors in the skin, joints, and muscles, including the primary spindle afferents. The A-beta and A-delta fibers are primarily cutaneous afferents. Group B fibers are preganglionic autonomics. Group C fibers include postganglionic autonomics, general visceral afferents, and pain and temperature fibers. The I/II/III/IV system applies only to afferent fibers. Groups I to III are myelinated; group IV is unmyelinated. The Ia fibers are spindle afferents from nuclear bag fibers; the Ib fibers arise from Golgi tendon organs; and the II fibers are spindle afferents from nuclear chain fibers. Group III fibers are cutaneous axons approximately the same as A-delta fibers. Group IV fibers correspond to C fibers and are primarily nociceptive.
In addition to the relationships between nerve fiber diameter, CV and sensory modality, the vulnerability to various types of injury varies with size and type of fiber. Cocaine, which blocks the conduction of the smaller fibers first, causes loss of sensation in the order of slow pain, cold, warmth, fast pain, touch, and position. Pressure, which blocks the conduction of the larger fibers first, causes loss of sensation in the order of position, vibration, pressure, touch, fast pain, cold, warmth, and slow pain. Most peripheral neuropathies affect both large and small fibers, but in some conditions, the involvement primarily affects either the large or the small fibers.
DERMATOMES
Sensory nerve roots supply cutaneous innervation to specific dermatomes. The dermatome innervation of the extremities is complex, in part due to the migration of the limb buds during embryonic development. As a result, the C4-C5 dermatomes abut T1-T2 on the upper chest, and the L1-L2 dermatomes are close to the sacral dermatomes on the inner aspect of the thigh near the genitalia. The generally available dermatomal charts are primarily derived from three sources: Head and Campbell, Foerster, and Keegan and Garrett, who all used very different approaches. Head and Campbell were primarily interested in herpes zoster and mapped dermatomes according to the distribution of herpetic eruptions. Foerster performed posterior rhizotomies in patients with chronic pain. He mapped the distribution of an intact root when one or more of those above and below had been severed or by electrically stimulating the stump of a severed root and observing the area of cutaneous vasodilation. The observation of dermatomal overlap originated partly from this work, and for a time, many believed a lesion of a single root would produce no detectable deficit. Keegan and Garrett examined a large series of patients with clinical involvement of various roots and mapped the sensory deficits; there was surgical correlation in 53% of the patients. The loss of sensation due to isolated involvement of a single root, as occurs clinically, produces a different dermatomal map than the preserved sensation in a zone of anesthesia as found by Foerster. It is clear that the dermatomal overlap is such that the clinical deficit from an isolated root lesion is typically much more restricted than that expected from the anatomical geography of the dermatome. Deficits to pin prick are smaller than those to light touch. Figure 36.5 shows the dermatome distributions as depicted by Keegan and Garrett.
ANATOMY OF THE POSTERIOR ROOT
The oval-shaped dorsal root ganglia (DRG) lie on the posterior root in the intervertebral foramen, just lateral to the point where the posterior root penetrates the dura. The connective tissue capsule around each DRG is continuous with the epineurium of the spinal root. The DRG is composed of neurons, satellite cells, and a highly vascular supporting stroma. The DRG neurons are unipolar. A single nonmyelinated “dendro-axonal” process leaves the cell and then bifurcates into peripheral and central branches. The peripheral processes conduct afferent impulses toward the cell body; they are functionally elongated dendrites but more closely resemble axons from a structural standpoint and by convention are referred to as axons. Large sensory neurons may be found singly or in small groups proximal or distal to the DRG.
Sometimes, the entire DRG lies in an ectopic intraspinal location, well proximal to its usual position, making it vulnerable to involvement by herniated nucleus pulposus or osteophytic spur. Such ectopic DRGs have been mistaken for tumors, with unfortunate results. The DRG for the C1 posterior root is often missing.
The dorsal root is divided into a medial zone, conveying large fiber proprioceptive traffic and a lateral zone conveying small fiber pain and temperature traffic. As the posterior root exits the DRG to enter the spinal cord, two discrete fascicles may be visible; these correspond to the medial and lateral divisions. After the posterior root joins the spinal cord, the pathways serving different sensory modalities diverge and follow very different central courses through the spinal cord and lower brainstem, only to draw closer together as they ascend through the upper brainstem to ultimately reconverge as they enter the thalamus.
CLINICAL EXAMINATION
Sensory function is divided clinically into primary modalities and secondary or cortical modalities. The primary modalities include touch, pressure, pain, temperature, joint position sense, and vibration. The cortical or secondary modalities are those that require synthesis and interpretation of primary modalities by the sensory association area in the parietal lobe. These include two-point discrimination, stereognosis, graphesthesia, tactile localization, and others. When the primary modalities are normal in a particular body region, but the cortical modalities are impaired, a parietal lobe lesion may be responsible. Itch and tickle sensations are closely allied to pain; they are probably perceived by the same nerve endings and are absent following procedures used for the relief of pain.
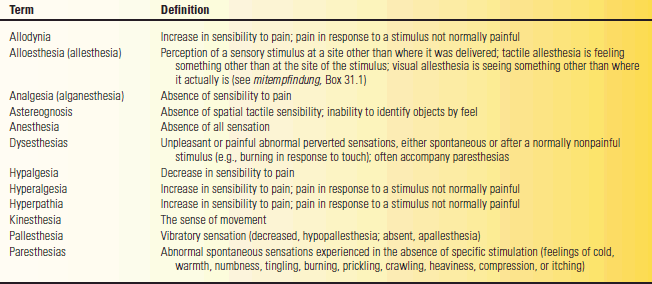
Many terms have been used, not always consistently, to describe sensory abnormalities. The definition of esthesia is perception, feeling, or sensation (Gr. aesthesis “sensation”). Algesia refers to the sense of pain (Gr. algos “pain”). Hypalgesia is a decrease, and analgesia (or analgesthesia) an absence, of pain sensation. The combining form “algia” refers to any painful condition. Hypesthesia is a decrease, and anesthesia an absence, of all sensation. Paresthesia is an abnormal sensation; dysesthesia (Gr. dys “bad”) is an abnormal, unpleasant, or painful sensation. Table 31.1 summarizes some of the definitions. Seldom-used terms and those of primarily historical interest are summarized briefly in Box 31.1.
TABLE 31.1 Generally Accepted Definitions of Commonly Used Terms Regarding the Sensory System and Abnormalities of Sensation