Pain
Pain and pleasure are the major driving forces of human behavior. We cover pain in this chapter but are ever so looking forward to discussing pleasure in the next chapter.
ACUTE PAIN
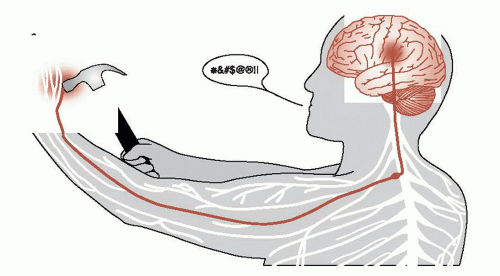
Acute pain, as we all have experienced, starts in the periphery, is relayed to the spinal cord, and then passes up to the brain where it produces a negative reaction (Figure 11.1). Pain-producing stimuli are detected by specialized afferent neurons called nociceptors. The nociceptors are free nerve endings—not an identifiable structure as we have for touch and vibration. These cells respond to a broad range of physical and chemical stimuli, but only at intensities that are capable of causing damage.
Peripheral Tissue
The nociceptors in peripheral tissue are activated by injury or tissue damage that results in the release of bradykinins, prostaglandins, and potassium (Figure 11.2). These molecules in turn cause the secretion of substance P from other branches of the axon, which stimulates the release of histamine and promotes vasodilation.
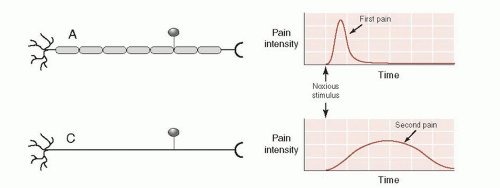
Aδ and C fibers send the signal to the dorsal horn of the spinal cord by way of the dorsal root ganglion. The Aδ fibers are myelinated axons that quickly send the first, sharp signals of pain. The C fibers are unmyelinated and send a slower, dull pain signal (Figure 11.3). It is the duller, slow pain signal of the C fibers that becomes so troublesome in chronic pain conditions (see box).
Spinal Cord
The nociceptive afferent nerve fibers synapse in the dorsal horn of the spinal cord. Information about tissue injury is passed on to the next neurons, which then cross to the contralateral side and ascend to the brain.
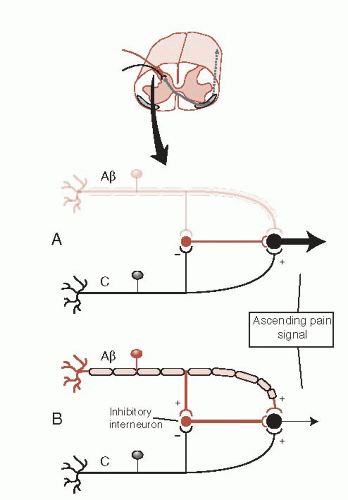
The signal can be modified at this point by descending fibers (discussed later) or from simultaneous activity by nonpain neurons (mechanoreceptors: Aβ fibers). The Aβ fibers can dampen the pain signal in what is called the gate theory of pain. Figure 11.4 shows how a signal from the larger mechanoreceptor activates an inhibitory interneuron in the dorsal horn, which results in a smaller signal conveyed to the brain. The gate theory explains why rubbing an injury seems to reduce the pain and is the rationale for the use of transcutaneous electrical nerve stimulation.
Ascending Pathways
There are a variety of ways to describe the different nociceptive pathways that ascend to the brain in the spinal cord. Unfortunately, there is no consensus on the proper nomenclature for these tracts and no two authors seem to use the same terms. Recently, the perception of pain—and the areas participating, in the central nervous system (CNS)—has been divided into two prominent domains: sensorydiscriminative and affective-motivational. This dichotomy (summarized in Table 11.1) is a convenient way to understand the ascending pathways.
Sensory-Discriminative
The sensory-discriminative domain encompasses the traditional sensory pathway taught in the first year of medical school. The signal travels up the spinothalamic tract, synapses in the lateral thalamus, and proceeds to the somatosensory cortex. Figure 11.5 is a diagram of this pathway. This type of pain allows the subject to become aware of the location of the pain and answer the question, “where does it hurt?” However, the perception of pain is much more than just identifying the location of a noxious sensation and withdrawing the injured limb.
Affective-Motivational
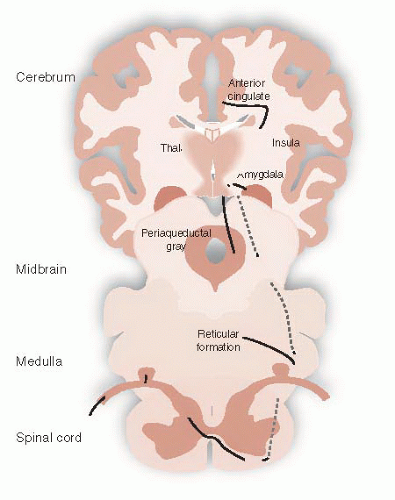
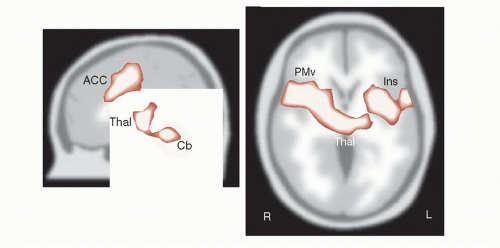
Other ascending sensory signals communicate the intensity of a noxious stimuli. There are several tracts that transport these signals, such as the spinoreticular tract or the spinomesencephalic tract— just to name a few. The important point is that all the signals travel in the anterolateral region of the spinal cord and terminate in different locations such as the reticular formation, periaqueductal gray (PAG) matter, and the amygdala. The rest of the signals synapse in the medial thalamus before proceeding to other areas of the cerebral cortex (Figure 11.6).
The affective-motivational signals communicate the unpleasantness of the sensation and answer the question, “how much does it hurt?” In the cortex, these signals activate areas associated with emotional feelings, such as the anterior cingulate cortex (ACC), insular cortex, and prefrontal cortex—as
well as the amygdala. Functional brain imaging studies over the past two decades have documented activity in these areas during pain perception— areas we typically associated with mood, attention, and fear. Activity in these regions of the brain helps us understand the concomitant depression, hyperfocus, and anxiety we see with patients in pain.
well as the amygdala. Functional brain imaging studies over the past two decades have documented activity in these areas during pain perception— areas we typically associated with mood, attention, and fear. Activity in these regions of the brain helps us understand the concomitant depression, hyperfocus, and anxiety we see with patients in pain.
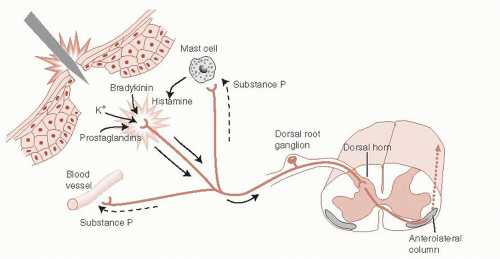 FIGURE 11.2 • Peripheral nociceptive responses to acute trauma. (Adapted from Kandel ER, Schwartz JH, Jessell TM, eds. Principles of Neural Science. 4th ed. New York: McGraw-Hill; 2000.) |
Figure 11.7 shows drawings of some of the areas that become active with acute pain. In this study, subjects were scanned at rest and later with a hot probe (approximately 50°C) applied to the upper right arm. Note the diverse areas that become active with acute pain—thalamus, ACC, prefrontal cortex, and insula, as well as others. The experience of pain is more than just identifying where it hurts.
It is also important to note that some of the activation is on both sides of the brain. This shows that there is more to the processing of pain signals in the CNS than implied by our simplified Figures 11.5 and 11.6.
TABLE 11.1 The Two Afferent Pathways Bringing Pain Signals from the Periphery to the CNS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
TREATMENT NEUTRALIZING C FIBERS
Over 20% of American adults struggle with chronic pain. The pharmaceutical industry is actively searching for an effective analgesic devoid of narcotic side effects or addictive potential. The transient receptor potential vanilloid 1 (TRPV1) receptor is one potential target. Found primarily on small diameter sensory neurons such as C fiber neurons, the TRPV1 receptor responds to a variety of noxious chemical and physical stimuli—including capsaicin, the active component of chili peppers. Animal studies suggest that blocking this receptor reduces the aching quality of chronic pain while preserving the sensations of touch and proprioception. The National Institutes of Health and several pharmaceutical giants are studying the effect of blocking the TRPV1 receptor in humans.
One concern regarding TRPV1 is the role the sensory neurons play in modulating inflammation. Activation of the TRPV1 receptor leads to the release of pro-inflammatory cytokines. Not surprisingly, animal studies have shown that TRPV1 disruption can impair bacterial clearance, cytokine response, and ultimately mortality from sepsis—another reminder that pain and protection are intimately connected.
CONGENITAL INSENSITIVITY TO PAIN
Congenital insensitivity to pain, a term used to describe rare genetic conditions in which people lack the ability to sense pain, initially sounds like a blessing but is actually a nightmare. Individuals with this condition fail to identify or respond to noxious, injuring stimuli and suffer excessive burns, fractures, and soft tissue damage. Ultimately, the unrecognized injuries and secondary complications lead to an early death.
The spectrum of congenital insensitivity to pain provides an example of the distinction between sensory and affective components of pain. Subjects with frank congenital insensitivity to pain are without the peripheral Aδ and C fibers. These patients
lack the sensory-discriminative as well as affective components of pain and are the most at risk for harm and premature death.
lack the sensory-discriminative as well as affective components of pain and are the most at risk for harm and premature death.
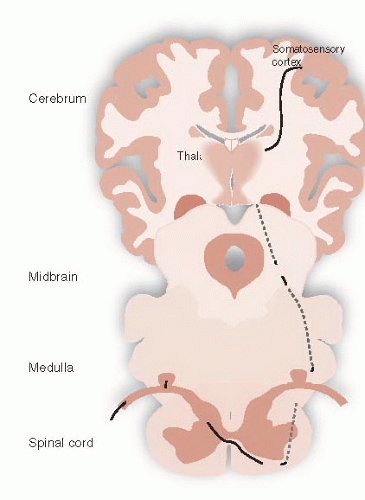 FIGURE 11.5 • Spinothalamic tract transmitting the sensory-discriminative pain signal from the periphery to the somatosensory cortex. |
A milder condition, termed congenital indifference to pain, is found in individuals who can distinguish sharp and dull pain, but are indifferent to the sensation. They lack the emotional responses and normal withdrawal movements; they can feel the pain, but are not concerned. Subjects with this disorder have normal peripheral nerve fibers but seem to have an as yet unidentified central impairment of the affective-motivational component of pain.
Congenital insensitivity to pain with anhidrosis is a specific rare autosomal recessive disorder characterized by absence of pain (along with inability to sweat, unexplained episodes of fever, and mental retardation). Patients with this condition have a mutation in the gene for the Trk receptor—the receptor that binds with nerve growth factor. As we saw in Chapter 8, nerves need nerve growth factor proteins to survive. Cells lacking the receptor are unable to incorporate the growth factor and wither away (or fail to develop). The patient lacks pain fibers and therefore does not experience pain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree