Chapter 125 Pain in Spine Disease
Back pain is one of the most common complaints in the general population; more than two thirds of the population have back pain at least once during life.1,2 Low back problems are reported to be the second leading complaint in outpatient consultations and the third complaint in hospital admissions.1,3 Annual back pain prevalence is reported to be 15% to 45%.4 Back pain is the most common cause of activity limitation in younger individuals,5 and it is the third most common cause of surgical procedures in the United States, in particular, fusion surgery.4,6 Although back pain is very common, 60% to 70% of patients with acute back pain are likely to recover in less than 3 months without functional loss. The prognosis worsens significantly when pain becomes persistent for more than 6 months, with less than 50% complete recovery.4 The recurrence rate is also higher in patients with persistent back pain.4 Many variables influence recovery, recurrence rates, and the probability of returning to work. Factors related to increased disability include gender (male), age, unemployment,7 stressful work environment,8 and compensation related to disability.7–10 Psychological factors also influence prognosis.11–15
Although less common than low back pain, neck pain is also a frequent reason for seeking health care. The most common causes of neck pain include musculoskeletal disorders and degenerative disease of the cervical spine.5,16 The lifetime prevalence of chronic neck pain ranges from 35% to 50%, and the cross-sectional prevalence is 10% to 35%.16–18
Most episodes of low back, neck, or related limb pain do not come to medical attention or are likely managed by primary care professionals. Specialized attention is most often required for severe, refractory, and chronic pain. However, even this smaller subsection of patients constitutes a large, imposing significant health-care challenge and burden to social security systems. The direct and indirect costs of chronic back pain have been estimated to be greater than 50 billion dollars in the United States,8 and chronic disability secondary to chronic back pain affects more than 5 million Americans8 (Fig. 125-1). This chapter focuses on chronic pain associated with spine disease, with emphasis on postlaminectomy syndrome and neuromodulatory treatment options.

FIGURE 125-1 Pain has been a subject of interest since ancient times.
(Copyright Cleveland Clinic Foundation.)
Chronic Pain
Definition
The transition from acute to chronic pain can be defined according to time course or healing process. The first criterion is more commonly used, although different cutoff time points have been arbitrarily chosen, ranging from 1 to 6 months.18A Chronic pain can also be defined as pain persisting beyond the expected time of healing for the given injury.19 This criterion avoids the need for an arbitrary cutoff but may not be as practical clinically. In this context, chronic pain is understood as pain that is not associated with tissue injury or illness of equivalent severity. Neuropathic mechanisms may be involved in pain perpetuation as well as the influence of behavioral, social, and cognitive factors.
Nociceptive Pain versus Neuropathic Pain
Nociceptive pain is associated with tissue injury, without compromise of the nervous system itself. It is mostly described as sharp, well-defined pain, localized over the injured area. Neuropathic pain may develop from persistent nociceptive pain secondary to continuous sensitization of the nervous system20 but can also occur as a result of injury directly to the peripheral or central nervous system. It usually consists of a less defined sensation, often described as burning, aching, or electrical shocks, and generally associated with altered stimulus perception, such as allodynia or hyperalgesia.
Pain-Related Pathways
Peripheral Receptors
When excited by a stimulus, the peripheral receptor acts as a transducer, transforming the initial information into chemical signals. Receptor depolarization is mediated by transmembrane potentials, triggered by external stimulation that reaches a specific threshold.20–23 Receptors are located on the endings of sensory axons in the skin and other tissues and are composed of free, partially covered or encapsulated nerve endings. Receptors are stimulus-specific and generally do not depolarize with other types of stimuli at normal intensities. Three main modality-specific receptor subtypes are associated with spinal pain: (1) nociceptors, which respond to tissue damage; (2) mechanoreceptors; and (3) thermoreceptors.21 These receptors include not only free nerve endings, as nociceptors, but also more specialized structures, such as partially covered receptors (Merkel discs, Ruffini endings) and encapsulated endings (Meissner, Pacini, and Golgi corpuscles; neuromuscular spindles; neurotendinous organs).21,23,24
Peripheral Nerve Fibers
Peripheral nerves are formed by the union of the dorsal root, which carries afferent information, and the ventral root, which contains mainly efferent information. The epineurium is formed not only by collagen and vessels but also by sympathetic fibers and polymodal receptors, forming the nervi nervorum.24 These are possibly associated with the occurrence of chronic pain after nerve injury,25,26 promoted by sympathetic sprouting and sensitization.
Nerve fibers are classified according to their myelinization and conduction velocity: Aα fibers are the fastest and are associated with muscle efferents. Aβ fibers are the second fastest type, carrying tactile, pressure, and proprioceptive afferents. These fibers are recruited during inflammation or other injury-related phenomena to participate in mechanisms of nociception, hypersensitivity, and sensitization.27,28 Aδ fibers carry not only cold information but also nociception when associated with polymodal receptors. B fibers are related to autonomic activity, and C, or unmyelinated, fibers are related to nociception transmission and postganglionic autonomic function.
Pain Pathways
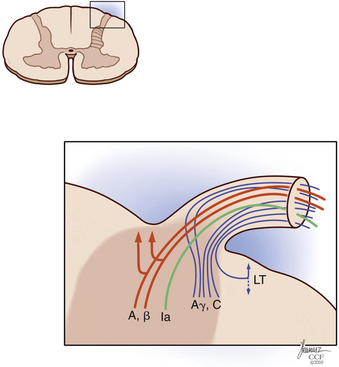
Painful stimuli are transmitted from the peripheral nerve to the dorsal root ganglion, dorsal root, and dorsal horn. At the level of the dorsal root entry zone, most unmyelinated and small myelinated fibers assume a more lateral position to enter the Lissauer tract (Fig. 125-2). In contradiction to the Bell-Majendie law, evidence exists that some of the nociceptive information travels not only through the dorsal root but also through the ventral root.29–33
The Lissauer tract comprises a bundle of longitudinal fibers and, as proposed by Ranson, is part of the pain transmission pathway.34,35 Unmyelinated fibers make up most of the Lissauer tract,35–37 and their central terminations are located mainly in lamina II of Rexed. Aδ fibers have a broader arborization and terminate in laminae I, II, V, and X.35 Although some Aδ fibers terminate in the dorsal horn at the same level they enter, others ascend many levels to terminate in higher segments of the cord.35,38,39
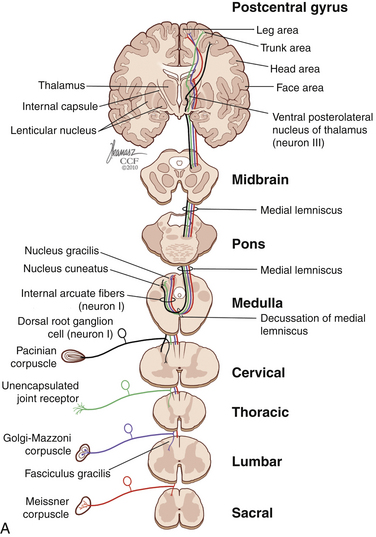
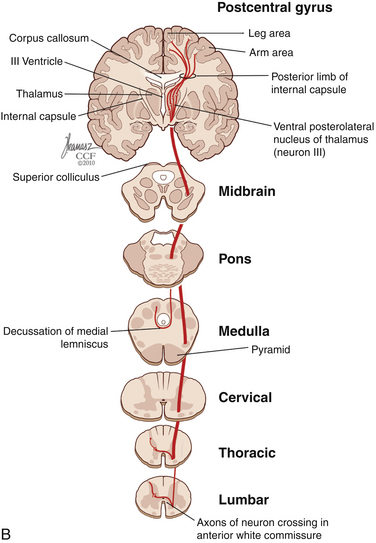
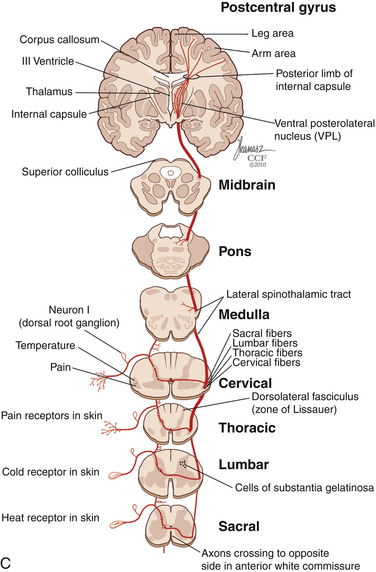
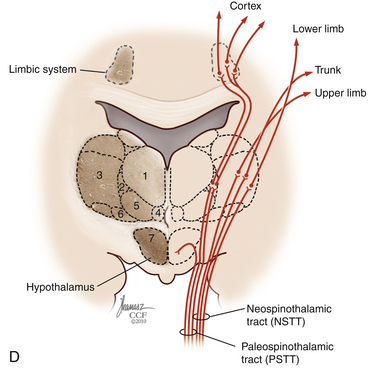
The dorsal horn is divided into ten laminae as defined by Rexed. Lamina I is related specifically to nociceptive and thermal information and is composed mainly of two types of cells: nociceptive-specific neurons, which respond to noxious stimuli, and wide dynamic range (WDR) cells, which respond to both noxious and non-noxious stimuli and are thought to be major contributors in the development of chronic pain.22,40 This lamina contributes to the formation of the spinothalamic tract (STT) (Fig. 125-3) and contains primarily substance P, calcitonin gene–related peptide, and enkephalin and serotonin as neuropeptides.22 Lamina II, also known as the substantia gelatinosa, receives nociceptive, thermoreceptive, and mechanoreceptive input.40 Cells in this lamina project to laminae I, III, and IV41 and contain opioid receptors,40 corroborating the importance of lamina II in modulating nociceptive information.22,42 Lamina III receives inputs from Aβ fibers and mechanoreceptive Aδ fibers.22 The sprouting of the low-threshold terminals present in this layer to the more superficial laminae,40,42 which are generally associated with nociception, suggests a role in chronic pain.5,43 Lamina V is another important component of nociception because of its inputs from Aδ and C fibers and WDR neurons, contributing to the formation of the STT.42,44
The cell projections of laminae I and V, after crossing the anterior aspect of the central canal, course through the STT in the contralateral ventrolateral column to reach the ventroposterior thalamus.40 Fibers of laminae I, VII, and IX, related to WDR neurons, project to the nonspecific intralaminar nuclei45–47 and to the brainstem reticular formation,48,49 periaqueductal gray matter,50–52 and hypothalamus,53 forming the paleospinothalamic tract22 (see Fig. 125-3). Because the WDR neurons have larger receptive fields and respond to different kinds of stimuli when compared to the specific nociceptive neurons, they are involved in poorly localized and nondiscriminative types of pain, in addition to the transformation of acute pain into chronic pain syndrome.22,52
After thalamic processing, pain information is projected to the primary somatosensory cortex and secondary somatosensory cortex sequentially.22,40,54 The thalamus also projects to the insula20 and the anterior cingular cortex,20 which are primarily related to the motivational and affective spheres of chronic pain22 (see Fig. 125-3).
The role of descending pathways in pain modulation is well established,55 starting in the periaqueductal gray matter, rostral ventromedial medulla, and dorsolateral pontine tegmentum.20,56 The periaqueductal gray matter receives inputs from the dorsal horn, brainstem, diencephalic system, and cortex57,58 and sends inhibitory projections to the dorsal horn.22 It also projects back to the thalamus and orbital frontal cortex,59 possibly exerting an ascending control of nociception. Another important pathway that plays a significant role in spinal pain modulation is the noradrenergic system,60,61 which projects extensively to the dorsal horn (Fig. 125-4). The development of chronic pain is related not only to ascending pain-facilitating mechanisms but also to reduced pain inhibition from descending and ascending modulatory mechanisms.
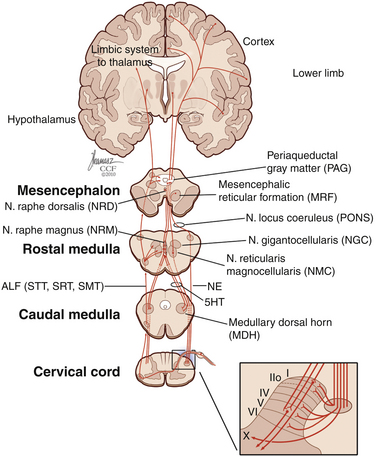
FIGURE 125-4 Diagram of descending inhibitory pain pathways and their respective projections in the dorsal horn.
5HT, serotonin; ALF, anterolateral fasciculus; NE, noradrenaline; SMT, spinomesencephalic tract; SRT, spinoreticular tract; STT, spinothalamic tract. (Copyright Cleveland Clinic Foundation.)
Physiology of Pain
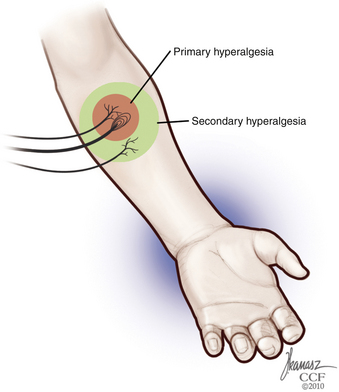
Each nerve fiber has different physiologic response durations, known as adaptation. Because C fibers are usually slowly adapting, and their responses last longer than the stimuli, the occurrence of temporal and spatial summation of painful stimuli during tissue injury may occur.24 Properties of other fibers include a well-defined receptive field and spontaneous discharges, generated without exogenous stimuli. Summation, expansion of the receptive field, and increase in spontaneous discharges are significantly enhanced during inflammation, leading to the development of hyperalgesia and sensitization.62 In addition to this mechanism of primary hyperalgesia, secondary hyperalgesia—increased pain sensitivity and allodynia in the surrounding uninjured area—may also occur, secondary to peripheral and central events, such as increased response to glutamate and central neuronal plasticity63 (Fig. 125-5).
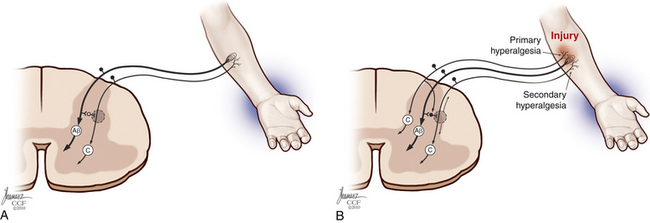
Each sensory cell has specific thresholds to respond to a given stimulus, which is lowered during inflammation. This condition is defined as sensitization, which is divided into peripheral and central according to the mechanisms involved.64 Peripheral sensitization is characterized by a decreased threshold20 and increased response to suprathreshold stimuli,65 spontaneous nociceptive neural activity, and expansion of the receptive fields after tissue injury66,67 (Fig. 125-6). The threshold of nociceptors is decreased as a result of exposed free nerve endings that fire abnormally. Sprouting of nerve terminals also generates ectopic discharges, as seen in neuromas.22
The increase in the number of sodium channels seen in damaged fibers68 may lead to nociceptor hyperexcitability, which is reverted at least partially by sodium channel blockers.69 Sympathetic sensitization is also a contributor,20 with increased nociceptor response to catecholamines of injured70,71 and uninjured neurons.72,73 The sensitivity of polymodal WDR receptors is also increased in response to inflammation, causing activation that is no longer triggered preferentially by nociceptive stimuli but also by other mechanical stimuli.20,63,64,74 Chemomediators are also involved in peripheral sensitization, with the secretion of cytokines interleukin-1β,75 interleukin-6,76 and tumor necrosis factor-α77,78 by lymphocytes, macrophages, and mast cells. Glutamate levels are increased during inflammation via macrophage and epithelial cell release, activating nociceptors via ion channels and metabotropic receptors.79–81
Central sensitization is mediated by short-term and long-term changes in the dorsal horn of the spinal cord.82–85 This mediation is supported by the occurrence of allodynia in association with the recruitment of Aβ fibers and their sprouting from lamina III into lamina II and loss of regulation of nociceptive fibers.22 Repetitive stimulation of C fibers, triggered by tissue injury, can lead to hyperexcitability and overactivity of these fibers and further perpetuation of nociceptive transmission.22,86,87 This perpetuation of nociceptive transmission can result in magnification of the sensory input and consequent expansion of the receptive fields,84,88–90 a phenomenon known as wind-up91–93
A key component in the development of chronic pain is antinociception and the failure of its underlying mechanisms. The inhibitory control of pain pathways is exerted by the spinal cord via several neurotransmitters, in addition to the descending inhibitory system as discussed previously.22 The most common inhibitory neurotransmitter in the spinal cord is gamma-aminobutyric acid, which mediates presynaptic inhibition of afferents,94,95 decreased release of neuropeptide P, and postsynaptic inhibition of the STT.42 Another important inhibitory pathway is the opioid system, with neurotransmitters that bind to three main receptors: mu, the most common opioid receptor in the spinal cord, which has the highest morphine affinity and mediates not only analgesia (μ1) but also respiratory depression (μ2)22,96,97; kappa, which binds to dynorphin; and delta, which binds to enkephalins.42 Opioids exert their action via presynaptic and postsynaptic mechanisms,98,99 and although they are well known for their analgesic effect, the symptoms caused by central sensitization, such as allodynia, usually do not respond well to these substances.
Cognitive and Behavioral Considerations in Chronic Pain
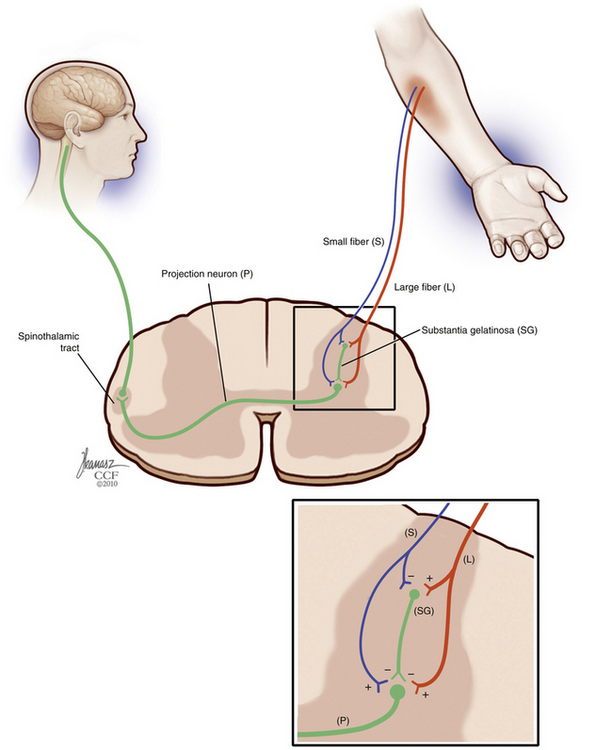
The gate control theory by Melzack and Wall in 1965100 is a landmark in the understanding of chronic and neuropathic pain. The gate control theory suggested that pain is not merely transmitted by the peripheral nervous system to the CNS and proposed instead endogenous modulatory mechanisms. According to this theory, pain transmission is modulated by a gating mechanism in the dorsal horn composed of large-diameter and small-diameter fibers that close (inhibit) and open (facilitate) the pain gate.
In 1999, Melzack101 proposed the neuromatrix theory, refining the gate control theory with additional key elements in pain processing. In the neuromatrix model, pain modulation occurs not only at the spinal level; cerebral mechanisms of pain processing and transmission are also taken into consideration, and cognitive and affective inputs are recognized to influence the final pain experience (Fig. 125-7). Several studies have corroborated further the role of cognitive and limbic systems in central pain processing.102–105 A patient’s beliefs and understanding about his or her pain syndrome, pain sensitivity, fear, anger, depression, anxiety, and catastrophic thinking influence the final pain experience.102,106,107 Patients who are able to develop techniques to cope with pain and reduce the impact of psychological comorbidities generally have a better prognosis.108–114
Based on these advances in understanding of central pain processing, the evaluation of patients with chronic pain should include not only a measure of the sensory component of pain (e.g., verbal, numerical, or visual analogue scales) but also the affective and evaluative components. The McGill Questionnaire has been largely validated as a comprehensive tool for the assessment of various chronic pain conditions, including spine pain.115 Other inventories can also be used, such as the pain disability index.116,117 Quality-of-life measurements such as the European Quality of Life Inventory may also be useful in evaluating the impact of the pain syndrome on the patient’s life and in assessing outcomes after interventions.
Work disability also plays a key role in the perpetuation of chronic pain, and its impact on long-term prognosis cannot be underestimated. Although the American Medical Association defines disability as “an alteration of an individual’s capacity to meet personal, social, or occupational demands…because of an impairment,”118 work disability agencies use more restricted definitions to guide benefit eligibility or ability to work.119 Compensation for disability and unresolved litigation have a complex influence on treatment outcome. Compensation and litigation have been linked to a poor prognosis,9,120–123 higher risk to develop chronic pain and pain behavior,124,125 and a lower likelihood of returning to work.126,127 Waiting for litigation and benefit disputes to resolve before discussing invasive pain interventions may improve outcomes and obviate the need for intervention.121,128 Likewise, patients who show little interest for becoming more active or returning to work may be less likely to benefit from additional intervention.129–131
Postlaminectomy Syndrome
Low back pain and pain of spinal origin are among the most common chronic pain conditions in the population132–135 and are associated with physical and psychosocial dysfunction, disability, and reduced quality of life. Although low back pain is more prevalent, neck pain is also a common reason to seek health care. Although approximately 90% of low back pain cases are nonsurgical,136,137 the proportion of patients undergoing spine surgery has progressively increased,6,138,139 and success rates remain variable (23–83%).140–142 Patient selection is known to be a key factor for successful outcomes, and inappropriate indications are likely associated with a higher frequency of postlaminectomy syndrome.139,143–145
Postlaminectomy syndrome, also known as failed back surgery syndrome (FBSS), is characterized by persistent, recurrent, and chronic back pain with or without radiation to the lower extremities after surgical treatment.20,134,146 The syndrome comprises different clinical etiologies138 and can occur after any surgical procedure, with or without fusion or instrumentation.138 Identification of the etiology of FBSS may assist in directing treatment, which includes medical, rehabilitative, surgical decompression or fusion, and neuromodulatory options.147 FBSS can be associated with numerous etiologies, including ruptured discs and fragments, which are found in approximately 15% to 35% of cases138,147,148; degenerative changes in levels adjacent to instrumentation149–153; extensive fusion associated with flat back syndrome154,155; pseudarthrosis156; and instability caused by facet joint failure after decompression.142,156 Some FBSS etiologies are associated predominantly with persistent leg pain.
Surgical treatment of spinal stenosis has failure rates of 10% to 30%.138,147,148 Accurate diagnosis and meticulous decompression may reduce the risk of FBSS.147 Foraminal stenosis, either residual or worsened by instability,142,155,157 is responsible for a large proportion of FBSS cases, followed by lateral and central stenosis.138,147,158 Recurrent or residual disc herniations causing nerve root compression are another common cause of persistent leg pain, with a highly variable incidence ranging from 10% to 50%.138,147,148 Neuropathic pain caused by prolonged dorsal root ganglion or nerve injury is thought to be less common but may lead to severe and refractory chronic pain.138,147,148 Arachnoiditis and epidural fibrosis, which can be caused not only by surgical manipulation but also by recurrent irritation and instability, may also lead to persistent pain.138,142,147,148
Although there are several alternatives for the management of FBSS, treatment is often challenging. It is common for patients and physicians to be disappointed and frustrated with outcomes. Patients may have already undergone multiple failed interventions, contributing to their psychological distress and reducing the odds that additional treatments will be successful. It is important to discuss treatment expectations beforehand with the patient and family. It is unlikely that additional treatments would be able to resolve completely chronic pain that has been refractory to medical management and surgery. In the setting of chronic back pain, a 50% improvement in pain is usually considered a reasonable outcome.159 Good candidates for any treatment for FBSS should be motivated to return to work and have a physically active lifestyle.159 A multidisciplinary approach has been recommended in the management of these difficult conditions, including physical therapy, rehabilitation programs, pain management, and possibly surgical intervention.160
Medical Management
Defining a logical algorithm is a critical step in chronic pain treatment, and conservative and reversible treatments are generally instituted first. Because medical management already has been covered elsewhere in this book, it is briefly discussed in this chapter. In this first approach, multidisciplinary assessment may allow for addressing the pain and its etiology and the management of comorbidities, such as depression and other psychological aspects of chronic pain, rehabilitation, and pharmacologic options.161 Back pain can be associated with modifications in neuromuscular activity, altering abdominal and back muscle function and contributing to the maintenance of the pain state.162–165 Physical therapy and rehabilitation are mainstays of low back pain treatment, and patients with FBSS should consider these programs, which are aimed at reducing biomechanical deficits and restoring strength and range of motion.162,166–170
The most common medications used for treatment of low back pain are nonsteroidal anti-inflammatory drugs, muscle relaxants, opioids, benzodiazepines, antidepressants, and antiepileptic drugs.171–174 Nonsteroidal anti-inflammatory drugs can be effective for acute and chronic pain,161,171,175,176 although long-term use of these agents is generally limited by side effects.
Muscle relaxants and benzodiazepines act predominantly on pain states perpetuated by muscle spasms and increased muscle tone and can be beneficial in the management of acute pain171,173,177; long-term use for chronic pain is not well established.161,171,178 Additional care has to be taken with benzodiazepines because of the risk of aggravating depression further and exacerbating the baseline condition.179,180
The use of opioids in the treatment of chronic nonmalignant pain is controversial and variable.171,181–185 Long-term use of narcotics has been linked to tolerance, addiction, and cognitive decline, which emphasizes the need for cautious use of these medications as a long-term option.184 Their analgesic effects are dose-related and are produced in various levels of the CNS, including the substantia gelatinosa of the spinal cord, the descending antinociceptive system, and the limbic system, altering emotional response and pain behavior.186–188 Neuropathic pain and allodynia are usually not responsive to opioids because of the activation of N-methyl-d-aspartate receptors (increased during central sensitization), leading to phosphorylation and inactivation of the opioid receptor.22 In this context, the use of opioids in the treatment of nonmalignant pain is more likely beneficial for patients with mainly nociceptive pain that did not respond to other medications, without associated major psychosocial comorbidity.189 Major long-term complications of opioid use include physical dependence,190–192 tolerance,184,185,190 addiction,184,193 opioid hyperalgesia,190,194,195 and cognitive dysfunction.184,196–199
Antidepressants are commonly prescribed in the management of chronic pain. Although they provide significant pain relief in various chronic pain states,200–203 studies in chronic low back pain indicate variable efficacy.204–207 The mechanism of action relies on activation of norepinephrine descending brainstem pathways, which is potentiated by serotonin (5-hydroxytryptamine) pathways.208 Tricyclic antidepressants such as amitriptyline and imipramine that block the reuptake of norepinephrine and 5-hydroxytryptamine in addition to H1, adrenergic, and cholinergic receptors tend to be the most efficacious.208–211
Anticonvulsants such as carbamazepine, topiramate, gabapentin, and pregabalin are also frequently tried in the medical management of neuropathic pain.212–215 Improvements in pain scores were seen with the use of these medications,216 mainly when radiculopathy was present,214,217–219 although patients with axial symptoms may also have some benefit.215 Anticonvulsants work primarily via suppression of abnormal discharge at nerve injury sites by Na+ channel block.220,221
Invasive and Surgical Management
Percutaneous Procedures
Patients with FBSS and low back pain caused by ruptured discs or facet joint instability may undergo provocative discography or medial branch block, although the diagnostic usefulness of these methods remains operator-dependent and controversial.222–225 Minimally invasive techniques can also be considered alternatives for the management of chronic pain related to FBSS. Procedures such as intradiscal electrothermal therapy and medial branch lesioning may provide functional improvement.223,226–234 Patients with predominantly leg pain may benefit from nerve root blocks as a guide for subsequent treatments.223,235,236 Pulsed radiofrequency lesioning of dorsal root ganglion has shown promising results in the treatment of radiculopathy associated with low back pain.237–239 Epidural injections, performed alone or in association with spinal endoscopy, have variable long-term efficacy in this population.223,240–245
Reoperation
Reoperation for FBSS in the absence of a clearly defined anatomic cause is a controversial option and may not provide significant improvement of the baseline condition.140,142,144,157,161,246 Success rates of reoperations in patients with FBSS may vary from 22% to 80% and depend on time of follow-up.161 Usual indications to consider additional back surgery include reasonable evidence of a surgically treatable condition (instability, compression of a nerve root, or cauda equina), clinical presentation that is compatible with the anatomic lesion, failure to improve with adequate conservative treatment, and management of surgical complications that may need urgent treatment.144,157,161 Back pain not associated with radicular symptoms, poor outcome after the first procedure, pseudarthrosis, epidural fibrosis, and psychological comorbidities all are factors associated with a worse prognosis.140,144,145,246,247
Intraspinal Delivery Devices
A significant proportion of patients with FBSS can fail to achieve pain relief with the previously discussed treatment options and with combined treatment. The use of long-term intrathecal infusion of pharmacologic agents can be considered an alternative in these cases.248–252 The advantage of this route of administration is in the proximity of the drug to the receptor as it diffuses passively in the dorsal horn, allowing for lower effective doses and consequently a reduced rate of side effects.250,252,253 Commonly used agents used include opioids, ziconotide, local anesthetics, and α2 agonists.250 Clinical trials are under way to evaluate the safety and efficacy of other agents for intrathecal infusion.254–256 Although long-term intrathecal infusion of opioids is already well established for treatment of malignant pain,249,251,257,258 the indications for nonmalignant pain are still controversial.249,259,260 The mechanism of action may include a direct inhibition of dorsal horn cells or a modulatory effect on interneurons, blocking the central transmission of nociceptive information.261–264 Several studies have shown promising results with short-term outcomes, even in patients with severe and refractory pain.249,252,265–270 However, studies with long-term follow-up often describe a decrease in responsiveness over time, without success in recapturing the same extent of early benefits.256,259,271–273
Appropriate patient selection is an essential step for satisfactory results and includes symptoms that are refractory to less invasive therapies, response to oral doses of opioids, significant response to intrathecal opioid trial, absence of addiction history, and favorable psychosocial evaluation.249,260,274–277 The option for intrathecal opioids may be more logical in elderly patients, for whom the goal is to achieve some degree of pain alleviation in the final years of life. Long-term management is more complicated in young patients, who are likely to increase the opioid dosage gradually over decades of use.
The intrathecal trial can be performed with sequential injections of bolus dosing with progressively increasing doses or continuous opioid infusion with an externalized catheter.249 During the trial, the fundamental goals are to assess efficacy in alleviating the chronic pain syndrome, potential side effects, and dosage. Permanent implantation of a pump and catheter system can be considered when a significant improvement is achieved (generally ≥50% reduction in pain) with tolerable side effects.249,250,259,274
Different opioids may be used for intrathecal delivery; these include morphine (approved by the U.S. Food and Drug Administration [FDA]), hydromorphone, fentanyl, and sufentanil.252,269,273,278 Their analgesic potential and side effects are strongly dependent on lipid solubility, and this should be considered when choosing the most appropriate drug for each patient.188 Hydrophilic drugs, such as morphine, may take longer to alleviate the pain, but the concentration remains high in the cerebrospinal fluid for longer periods, allowing it to ascend to supraspinal levels, enhancing the analgesic effects.249 Lipophilic drugs, such as fentanyl and sufentanil, have a rapid onset of action and prolonged duration but do not diffuse easily in the cerebrospinal fluid.249,279
The most common side effects described with intrathecal opioids include nausea, sedation, confusion, pruritus, urinary retention, myoclonus, reduced libido, and respiratory depression,259,271,273,280 which are greater with intrathecal hydrophilic drugs.249 Peripheral edema, usually unresponsive to diuretics, is another side effect of morphine and can be managed by changing to a lipophilic agent.281–283 Granuloma formation is possibly the most serious long-term adverse effect of intrathecal therapy.284 Although different agents have been related to this complication,279,285 it seems to be more common with higher concentrations and doses.279,286 In addition to routine imaging, screening of patients with intrathecal pump systems has been suggested to enable early detection of masses associated with the catheter tip. A panel of experts has recommended that clinicians managing these patients maintain a high index of suspicion and a low threshold for requesting imaging examination aimed at identified granulomas. Imaging should be considered not only for patients with progressive neurologic deficits but also for patients with subjective changes in neurologic status or loss of efficacy to the intrathecal infusion. Treatment options for catheter tip inflammatory masses include replacing the solution for saline and careful observation of neurologic progress. Patients with neurologic deficits or worsening neurologic examination may need surgical intervention aimed at decompression and removal of the hardware.286–289
Ziconotide, a blocking agent of N-calcium channels, is an intrathecal drug approved more recently by the FDA for chronic pain management. Its efficacy has been shown in the literature,290–294 but the high incidence of serious side effects during the dose titration phase may limit its use.279 The most common side effects include memory impairment, confusion, hallucinations, dizziness, and ataxia.290,291,294 Second-line drugs are indicated for opioid-resistant patients and include local anesthetics and α2 agonists, which act mainly on the neuropathic component of pain, enhancing analgesia.256 Bupivacaine and clonidine are generally used in combination with morphine and have been shown to restore pain control and improve quality of life.248,256,295
The major technical complications related to intrathecal pumps include infections involving the implanted hardware, postural headaches, cerebrospinal fluid leak, pseudomeningoceles, seromas associated with the pump reservoir, and device-related complications. Although the pumps have an expected expiration time, catheter complications are more commonly the cause of premature failure of the implanted system.296 Spinal cord injury associated with implantation of the catheter has been reported,297 but its frequency, although thought to be low, has not been established.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree