FIGURE 5-1 Superimposition of grand-averages of LEPs during waking and stage 2 (A) and REM sleep (B). For both, Left: scalp distribution of LEPs. Right: traces obtained at Fz (top) and Pz (bottom) during waking (gray) and stage 2 or REM sleep (black). Note that during stage 2 the response contains a negative late wave of K-complex on Fz and that on Pz N2/P2 is present with decreased amplitude. During REM sleep the response is virtually absent on Fz while it persists with reduced amplitude on Pz. C: 3D activity maps at the latency of the P2 component during waking (left) and REM sleep (right). D: Grand average LEPs in referential recording mode during waking, sleep stage 2, and REM sleep in the operculum (bottom), the insula (middle), and the mid-anterior cingulate (top). On the left part of the figure, for each structure, the contacts where the maximal amplitudes of the C1–C2 components were recorded are indicated. Note the depressed response in the cingulate during REM sleep. LEP, laser-evoked potential; REM, rapid eye movement. (Adapted from Bastuji et al. [2, 3].)
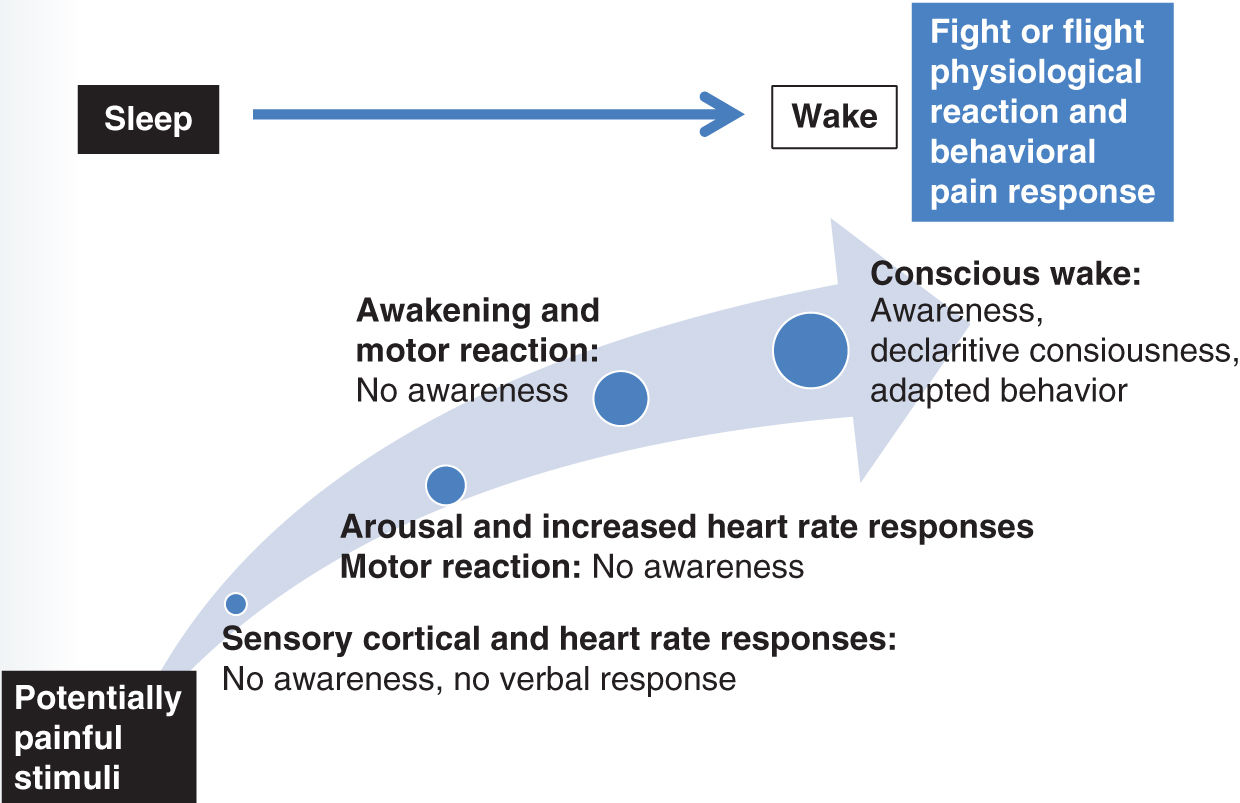
FIGURE 5-2 Crescendo of physiological response from unconscious nociception to consciousness of pain.
Nociceptive Reflex
The pain withdrawal reflex, classically used to behaviorally assess pain sensitivity, is an involuntary action aiming at taking away the part of the body receiving the nociceptive stimulus. This reflex can be mainly mediated at the spinal level (as the tail flick) or involve an upper brain motor response (hind paw withdrawal to paw lick).
Studies performed during sleep in animals provide contradictory results. In cats, the nociceptive tail flick reflex latency to radiant heat was increased (analgesic response) in non-REM to REM sleep as compared to wakefulness [19]. In rats, the hind paw withdrawal latency was found unchanged with laser heat across sleep/wake states but, surprisingly, was shorter (algesic response) during slow-wave sleep than during waking when light radiant heat was applied [27]. Despite the fact that rats remove their hind limb from noxious heat during sleep, and then quickly return to sleep afterward, these findings support that during sleep nociception remains an active protective process to preserve body integrity. In humans, the polysynaptic nociceptive flexion reflex threshold (R3 reflex) has also been used to assess nociception during sleep. The R3 reflex is induced by an electrical stimulation of a nerve and its threshold, amplitude, and duration are outcome measures. In comparison to wakefulness, the threshold to evoke a R3 reflex was increased during stage 2 and also in deep NREM sleep; it was even higher during REM sleep [39]. The amplitude and duration of the R3 reflex were higher during REM sleep than during wakefulness, suggesting a facilitating effect of REM sleep on spinal polysynaptic nociceptive reflexes. Thus, a paradoxical mechanism on one side preserves sleep continuity, and on the other side keeps a protective mechanism to prepare a reactive behavior, a “flight or fight” response.
Quantitative sensory testing (QST) protocols using thermal, mechanical, and chemical models of experimental pain have been also used to observe motor reactivity during sleep in humans. With a painful heat thermode [23] or laser [3] stimuli, a nocifensive behavioral–sensory–motor response was reported to occur during all sleep stages, and is more likely to occur in association with a sleep arousal (brief heart, brain, and muscle activation); these nocifensive responses were observed in the absence of cortical or behavioral arousals, following 2.5–11% of the stimulations. Therefore, sleep-reflex behaviors in response to nociceptive stimuli can be observed without triggering a discontinuation of sleep and, interestingly, without inducing any hyperalgesia in the morning following the experimental sleep session [4, 10, 23].
Sleep Disruption by Nociceptive Stimuli
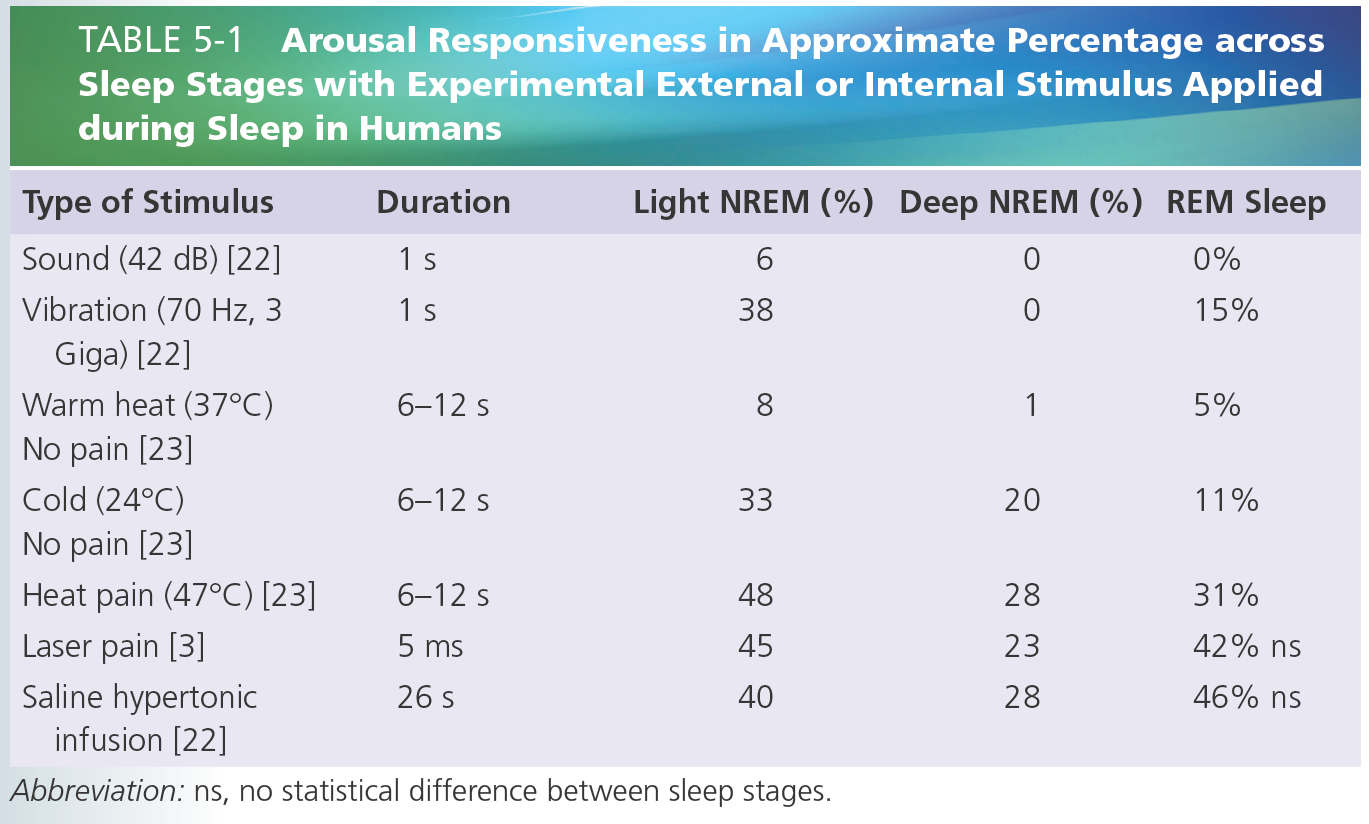
In case of painful stimulation, sleep disruption is frequently observed both in clinical and experimental conditions [3, 4, 12, 22, 23]. As described above, the responsiveness to sleep disruption can be considered as an important protective mechanism that prepares the individual to react to homeostatic-threatening stimuli by enhancing his or her level of consciousness. Thereby and as expected, somatosensory nociceptive stimuli tend to induce more arousals than non-nociceptive somatic stimuli [3, 23] or repetitive auditory stimuli [1, 22] (Table 5-1). Awakening thresholds may differ according to sleep depth, suggesting that nociceptive inputs are submitted to a state-dependent gating [44]. As previously reported with other sensory stimuli [5], the intensity needed to trigger an arousal response in deep NREM and REM sleep was also reported to be higher than that needed to do so during light NREM sleep [4, 10, 11, 23]. Nevertheless, such sleep stage dependence was observed with thermal and mechanical stimulations, but was lost with longer stimulus durations (hypertonic muscular saline injections) [22], or brief cutaneous laser stimuli [3]. These latter stimuli induced an equipotent frequency of awakening responses across all NREM and REM sleep stages (see Table 5-1). Reactions to muscle or joint stimuli have also been described to be of higher magnitude than those to cutaneous stimuli [11], and as expected the duration of sleep disruption is longer with sustained [22] than with brief painful stimuli [3]. This suggests that the relevance of these stimulations might activate a top-down mechanism to override the brain stem neurons responsible for sleep maintenance and, consequently, activate the ascending arousal system to drive an awakening. Nevertheless, as mentioned above, it is of interest to note that brief pain-related stimuli, all perceived as painful during wakefulness, were not reported to be perceived by healthy volunteer subjects during sleep [2, 3, 22, 23]. The incorporation of nociceptive stimuli into dream contents is also unusual [32] and might be more associated with stimulus-related unpleasantness and duration, rather than with intensity [22, 38].
INTERACTIONS BETWEEN PAIN, REM SLEEP, AND PLACEBO-INDUCED EXPECTATIONS OF PAIN RELIEF
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






