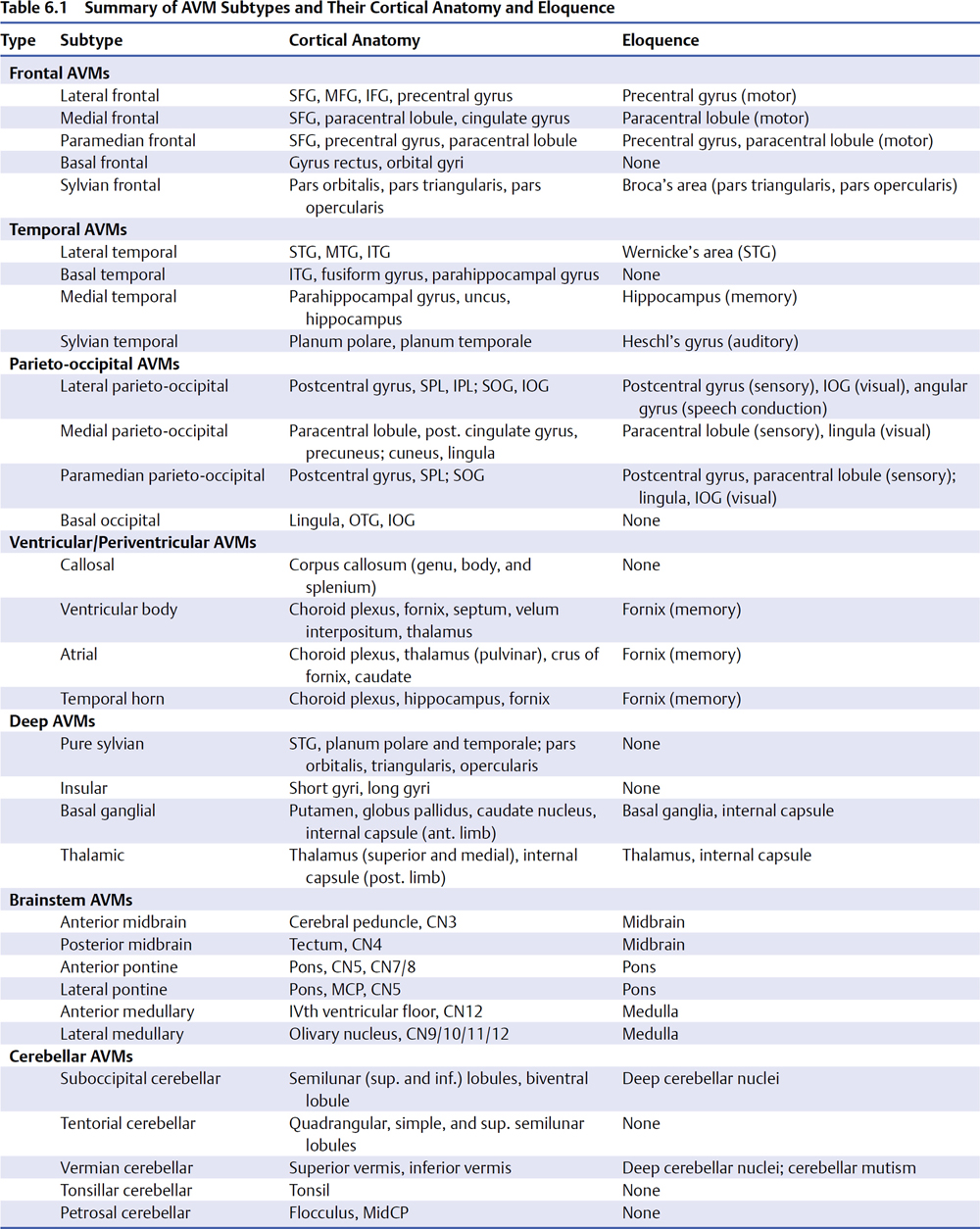
6 Parenchymal Dissection Parenchymal dissection detaches an arteriovenous malformation (AVM) from brain. Although pial dissection quickly exposes margins and closes superficial afferents, parenchymal dissection is lengthier and comprises two thirds of the circumdissection. Every piece of the AVM’s four sides is separated from adjacent brain, every intersecting artery is interrupted, and secondary draining veins are trimmed. An AVM with reduced flow is more cooperative during circumdissection, and therefore hot sides with significant arterial input are tackled first. Circumdissection should spiral around the nidus and progressively deepen the dissection plane rather than thrusting into narrow foxholes with poor visibility. Spiraling dissection gains perspective on the AVM’s shape and size, and enables retraction of the nidus. Feeding arteries in deep corners are alluring, but one should pause before engagement, zoom the microscope out, and deepen the dissection plane on other sides to optimize exposure before returning to the feeders, in case they bleed. Parenchymal feeders tend to be smaller and less ferocious than cortical feeders, but are harder to see. Shallow parenchymal dissection is performed with dynamic retraction by the bipolar forceps or sucker on the nidus or adjacent brain, but deep parenchymal dissection may require fixed retraction with self-retaining retractors. Blades are preferentially applied to the AVM rather than brain tissue, with a small piece of Nu-Knit on the nidus at the point of contact. Fixed retraction is usually kept to one retractor; multiple retractors or circumferential retraction systems build a ring around the surgical field that deepens and obstructs it. The tense dynamic between tight AVM adherence and wide circumdissection into brain, between aggravating the AVM and harming eloquent tissue, only increases during parenchymal dissection. Most AVM subtypes are associated with some eloquent anatomy (Table 6.1). Arteriovenous malformations have irregular contours formed by arterial networks, loops, veins, varicosities, and tortuosities that invite coagulation, but dissection should follow the interface plane and widen into adjacent brain. For example, with accurate interpretation of an arterial loop, dissection veers around this nidal protrusion and avoids needlessly interrupting the loop’s afferent and efferent limbs or inducing bleeding. Similarly with venous loops, accurate interpretation avoids inadvertently occluding outflow. The dissection course shifts constantly as the AVM margin is explored and scrutinized, migrating to and from the nidus. Vascular structures originating from the AVM are followed into brain and resolved as artery, vein, or additional nidus. Hemosiderin stains, gliosis, and encephalomalacia are followed to redefine the interface plane. Each advancing step must avoid misinterpretations that might cause AVM bleeding, remnants, damage to eloquent brain, and new neurologic morbidity. Arteriovenous malformation hemorrhage is a significant factor that facilitates AVM resection by separating portions of the nidus from adjacent brain. The rupture performs some parenchymal dissection. Hematoma evacuation immediately accesses this dissection plane as if it were a free cortical surface. Hematomas associated with ruptured AVMs are often large, and their cortical extension localizes AVMs that are not cortically based. Hematoma that presents on the cortical surface or discolors overlying cortex, or that can be identified with ultrasonography or stereotactic navigation, can lead to deep AVMs without a superficial draining vein. By definition, clot communicates directly with a ruptured AVM and clot evacuation will reveal it. Hematoma is evacuated strategically in areas remote from the AVM, deliberately avoiding the nidus until the cavity is cleared, the brain is decompressed, and landmarks such as draining veins, feeding arteries, and ventricles are identified. Overly aggressive hematoma evacuation may re-rupture the AVM without control of afferent arteries or clear orientation within the cavity. A tenuous cap of thrombus covering the rupture site on a draining vein or varix should be left alone. Feeding arteries traversing through clot are followed to the AVM nidus which usually resides in a wall of the hematoma cavity. The hematoma’s relationship to the AVM determines its surgical advantage: hematoma adjacent to nidus dissects its parenchymal sides; hematoma beneath nidus facilitates deep dissection of its ependymal side; and hematoma above nidus opens a transcortical corridor to its top side (Fig. 6.1). The latter relationship is particularly advantageous because trans-cortical routes to deep AVMs are nonanatomic and do not exist in patients with unruptured AVMs. Accessing an AVM through the hematoma cavity requires no corticectomy, produces no additional morbidity, and reaches AVMs in deep lobar white matter, basal ganglia, and thalamus.
 Circumdissection
Circumdissection
 Rupture and Hematoma
Rupture and Hematoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree