INTRODUCTION
Despite the relative success of potent antiretrovirals (ARVs) in reducing plasma HIV viral load and improving prognosis in HIV patients, there still remains an increased susceptibility for certain mental disorders in patients with HIV when compared to the general population. These disorders include HIV-associated neurocognitive disorder (HAND), major depressive disorder (MDD), dysthymia, generalised anxiety disorder (GAD) and panic attacks [1]. Although two main types of HIV exist (HIV-1 and HIV-2), the majority of HIV infections globally have been caused by HIV-1, with HIV-2 being largely confined to West Africa. Therefore, in the forthcoming sections to avoid confusion HIV will denote HIV-1. Although it has been postulated that patients with other serious mental disorders such as schizophrenia or bipolar disorder may have an increased risk for contracting HIV, this has not been found to be the case in a large longitudinal study of patients with HIV even when controlling for substance abuse [2]. Whilst ARVs suppress HIV RNA levels within the cerebrospinal fluid (CSF) [3, 4] detectable CSF viral load still persists in some patients with a suppressed plasma viral load [5, 6]. Furthermore, in a recent study of HIV viral escape within the CSF of subjects on ARVs, the relative proportion of neurologically asymptomatic patients with detectable CSF viral loads was 10%, much higher than that previously reported [7]. With HIV invading the central nervous system (CNS) very early on following primary infection, persistent detectable viral load in the CSF may be related to productive infection; however, it may also be related to latency amongst cells such as astrocytes that can harbour the virus. As these cells are capable of surviving lengthy periods whilst being infected, they may act as reservoirs for CNS HIV. This concept is supported by a recent study that found the proportion of astrocytes infected with HIV in brains of patients with HIV-associated dementia (HAD) to be up to 19% [8]. Importantly, this is much higher than what was previously detected or theorized. The reason for this stark increase is likely an increased precision of estimation brought about through techniques such as laser capture microdissection that was employed by the authors of this study. As CSF viral load can persist, understanding the mechanisms behind CNS infection, latency and how effective current ARVs are in reducing these parameters is important and has implications for preventing or reducing the spectrum of symptoms associated with HAND and the subsequent release of viral particles to cause damage to neuronal circuits. Therefore, strategies for reducing infection and latency may have a pronounced effect on reducing HIV-associated neurodegenerative changes that have been well characterized within the brains of patients with HIV [9, 10] and that are also likely contributors to HAND.
PSYCHOSOCIAL AND BEHAVIOURAL RISK FACTORS
Education, Ethnicity and Gender
Low educational attainment has been suggested to reflect an indirect index of lower cognitive reserve capacity that lowers the threshold for neuropsychological (NP) abnormalities in cases of early HIV infection [11]. In an Italian study of 90 HIV-negative, 88 asymptomatic HIV-positive and 94 symptomatic HIV-positive persons, individuals with less than 6 years of schooling had an odds ratio (OR) of 17.2 (95% confidence interval [CI], 3.6-83.3) for cognitive impairment, independent of age, sex, disease stage, ARV therapy and risk behaviour, compared to those with greater than 6 years of education [12]. In addition, multivariate prediction models that considered both sociocultural factors and CD4 count revealed that reading level was the only unique predictor of global NP functioning, learning and attention/working memory [13]. In some countries, it has been shown that ethnicity corrections significantly improved the correct classification of HAND in HIV-positive individuals of African American and Hispanic American background [14]. In other countries, the geographical background (i.e. rural vs urban) rather than ethnicity has more of a predominant effect on cognitive functioning in HIV-positive individuals [15], and, when taken into account, lead to a significant improvement in the correct classification of HAND. It is well known that some NP tests at the population level show gender effects (male vs female) [16]. Correction for gender is therefore advised on those tests to reach an optimal impairment classification in HAND. Moreover, in some economically emerging countries, gender, ethnicity and geographical background can significantly interact, and HIV-positive women are more likely to perform significantly worse than their male counterparts.
Methamphetamine and Alcohol
Several studies have demonstrated that intravenous drug use (IDU) as well as non-IDU of several psychoactive substances (e.g. methamphetamine (METH), cocaine, alcohol) are associated with an increased risk for HAND [17]. Amongst these psychoactive drugs, METH use is the most consistently associated risk factor for HAND. It has been suggested that drugs such as METH increase risky sexual behaviour and thereby may facilitate transmission of the virus [18, 19]. However, METH is also a comorbid factor for psychiatric disorders with HIV as it has been shown to cause neurotoxicity with HIV-positive METH users having a greater loss of interneurons within the frontal cortex correlating with greater cognitive impairment [20]. METH also interacts with HIV-Tat (transactivator of transcription) to induce synergistic neurotoxicity [21], and clinically HIV-positive METH users have been shown to have greater levels of cognitive impairment when compared to HIV-positive non-METH users [22]. In addition, prolonged exposure in rats results in the loss and/or atrophy of interneurons and pyramidal cells within the hippocampus together with an increased activation of microglia [23]. Alcohol has also been suggested as a risk factor for HAND, albeit reports have been conflicting [17].
Neuropsychiatric Disorders
Amongst the neuropsychiatric disorders, the most commonly studied and the most prevalent in HIV-positive individuals is MDD. Studies examining the effect of depressive complaints on neurocognitive functioning in HIV-positive populations have reported mixed results. Several studies have reported that HIV-1 infected individuals with a major depressive episode or self-reporting depressed mood on standard instruments may perform more poorly than non-depressed individuals in some cognitive domains (e.g. memory tasks) or report more cognitive complaints [24–26]. However, none of these studies reported an association with neurocognitive impairment. In contrast, others studies have suggested a possible link between increased depressive complaints and lower cognitive function [27–30]. However, a 2-year longitudinal study of 227 HIV-positive adult men who did not meet the criteria for a current major depressive episode at baseline reported no neurocognitive performance differences in association with lifetime or incident depression and suggested that neurocognitive impairment and major depression be considered two independent processes [31]. Still, because this study included only men and mostly mild forms of HAND, generalizability of the findings is not warranted. Fatigue has also been associated with depression and subjective cognitive complaints; however, fatigue was not shown to directly affect neurocognitive functioning [32]. Other relatively common neuropsychiatric disorders that may impact negatively on neurocognitive functioning in HIV-positive individuals are GAD, post-traumatic stress disorder and bipolar disorders. Bipolar disorder and/or psychotic symptoms (e.g. schizophrenia) amongst HIV-positive individuals is likely to significantly affect the NP profile and severity of HAND [33]. With regard to depression and other stressors affecting progression of HIV, it has been observed that such events can lead to increased viral load as well as a lowering of CD4 counts [34]. One such critical factor leading to a reduction of T lymphocytes and hence aiding in the progression of HIV is cortisol [35]. In addition, CD4 counts have also been found to be lower in individuals infected with HIV who have experienced post-traumatic stress symptoms and who scored lower on health-related quality of life (HQOL) scales [36].
HIV BIOLOGY AND REPLICATION
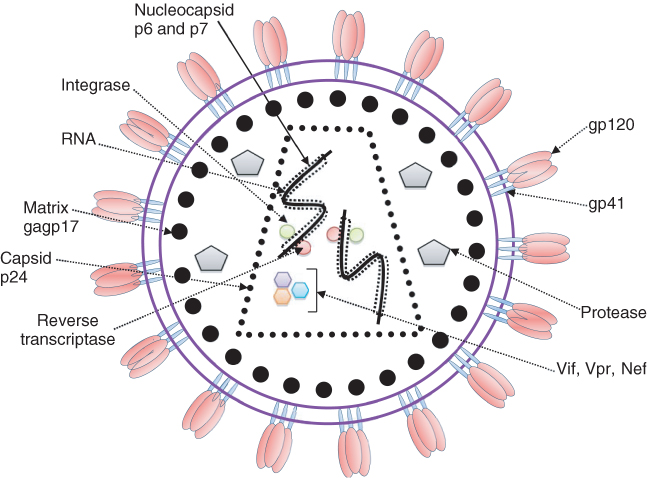
HIV is a lentivirus belonging to the family of retroviruses. It is comprised of two single strands of RNA that are enveloped and tightly bound to the nucleocapsid proteins p6 and p7 as well as the enzyme’s reverse transcriptase (RT) and integrase that are essential for the development of a functional virion. The capsid, containing the stable viral protein p24, surrounds the nucleocapsid, with a matrix containing another viral protein p17 encircling the capsid itself. This matrix is in turn surrounded by a phospholipid bilayer envelope in which the membrane-associated glycoproteins, gp120 and gp41, are embedded. The external domains of gp120 facilitate HIV entry into cells by promoting fusion of the viral envelope with the host cell membrane in a complimentary way. This is achieved through initial binding of gp120 to CD4, the main cell surface receptor required for viral entry. Binding of CD4 causes a conformational change in gp120, exposing its chemokine-binding domains to the HIV chemokine coreceptors CXCR4 and chemokine receptor 5 (CCR5), whose binding in turn stabilizes the docked virion. This added stability allows gp41 to penetrate the cell membrane and form a hairpin loop bringing the cell membrane and viral envelope into close proximity to one another and allowing internalization of the virion. Following entry to the cell, RT produces a DNA copy of the two positive RNA strands containing the viral genome, which is then actively transported together with other necessary viral proteins via the nuclear pore. Within the cell’s nucleus, the viral DNA is then incorporated into the cell’s existing DNA via the enzyme integrase and through interaction with nuclear factor kappa B (NFκB) to produce viral transcripts that are released into the cytoplasm and translated to proteins, including Tat, which promotes new viral production, and Rev, which allows the virus to leave the nucleus unspliced. Gag and Env (gp160) proteins that are produced from a full length HIV mRNA then associate and package the viral particles. They are then transported through the Golgi apparatus where Env is cleaved by a protease forming gp41 and gp120. This cleavage process is the target for protease inhibitors that are used in combination with reverse transcriptase inhibitors (RTIs) to inhibit viral replication and reduce viral load. Following cleavage of gp160, gp41 and gp120 products are then transported to the plasma membrane where gp41 anchors gp120. Gag (p55) and Gag-Pol (p160), which are produced from Gag, also associate with the plasma membrane of the host cell together with the two strands of HIV RNA encoding the viral genome. Together, they are then incorporated into the final new viron that buds from the host cell membrane and continues its journey to attach to another host cell and begin the replication cycle once again (Figure 2.1).
CNS ENTRY AND NEUROBIOLOGY
HIV enters the CNS very early on following initial infection, with detectable virus being present within the brain in 2 weeks [37]. This timeframe corresponds to a point where patients have not yet started receiving ARVs and as such affords the virus the ability to infect cells of the CNS for replication or to form reservoirs within cells such as astrocytes in order to lay dormant and help rebound viral replication later on. Within the CNS, HIV primarily infects cells through the binding of CD4 and CCR5, with this coreceptor being expressed on microglia and astrocytes but not on neurons. CNS replication of HIV is carried out either by microglia, the resident immune cells of the brain, or by infiltrating macrophages that cross the blood–brain barrier (BBB). Whilst astrocytes are capable of being infected, they are replication incompetent; however, as mentioned previously, the number of astrocytes that are capable of being infected can reach 19% and therefore infection of these cells represents a route for HIV to remain latent within the CNS. This latency may then contribute to HIV viral rebound if ARV regimens are ceased. Transmigration of macrophages across the BBB plays a key role in HIV neuropathogenesis. Over the last decade, we have made significant advances in understanding the mechanisms by which macrophages achieve this. Under normal conditions, macrophages extravasate through the BBB in a tightly regulated manner, through tight junctions that are formed by the contact of astrocyte end foot processes with brain endothelial cells playing an important role in this control. Under pathophysiological conditions, such as HIV infection, associated inflammatory responses and release of cytokines make these tight junctions more ‘leaky’, allowing a greater movement of macrophages across the BBB and into the brain. In relation to this effect, gp120 has been shown to increase permeability of the BBB and may represent one HIV-related mechanism for viral entry to the CNS. The main proinflammatory cytokines produced in response to HIV infection and responsible for increasing paracellular movement across the BBB are tumour necrosis factor-alpha (TNFα), interleukin 1-beta (IL-1β) and interleukin 6 (IL-6), with nitric oxide also playing a significant role in opening up the BBB. HIV-1 infected macrophages are capable of crossing the BBB by travelling through endothelial cells themselves (paracellular) or through the intercellular space between the endothelial cells (transcellular) [38].
Steps in the transmigration process include (i) capture from the circulating blood flow to the brain microvascular endothelial cell (BMVEC) membrane, (ii) rolling, (iii) firm arrest, (iv) intravascular crawling and finally (v) diapedesis through the BBB as illustrated in Figure 2.2 below.
Figure 2.2 Transmigration of HIV-infected monocyte through the blood–brain barrier.
Source: Adapted from Williams et al. [39].
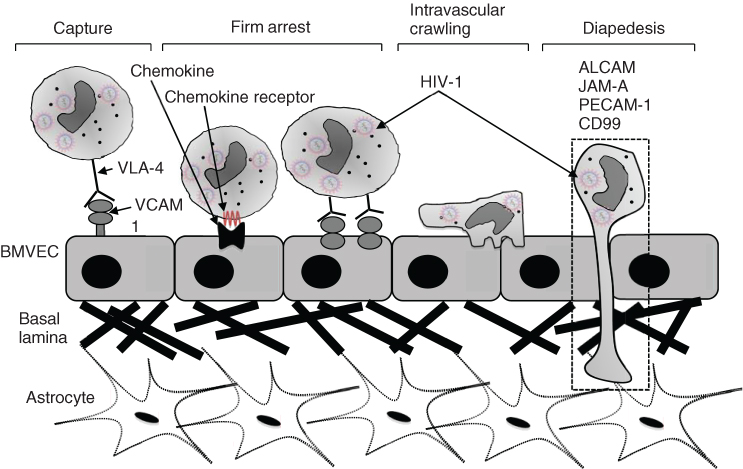
Diapedesis of monocytes across the BBB is mediated by a number of molecules including ALCAM (CD66), JAM-A (F11R/CD321), PECAM-1 (CD33) and CD99 [39]. Transmigration of monocytes across the BBB enables HIV establishment within the CNS as well as activation of neuroinflammatory pathways that have been implicated in HAND. These monocytes can then infect microglia, perivascular macrophages and astrocytes [8, 40]. Infected cells can then release HIV viral proteins such as tat and gp120, which in turn can activate macrophages, microglia or astrocytes to release proinflammatory cytokines. These cytokines include TNFα, IL-1β and IL-6. Accompanying this cytokine response, additional chemokines are also released, thereby serving to recruit further HIV-infected macrophages from the periphery into the CNS. This cycle perpetuates a chronic inflammation state and results in a more permeabilized BBB and astrocyte dysfunction, including glutamate dysregulation, which in turn can lead to excitotoxic damage to neurons and ultimately their degeneration and loss [41].
HIV NEUROTOXICITY
There is a growing consensus that documented changes seen in brains of individuals with HAND, such as axonal injury and synaptodendritic damage [42] and aberrant synaptic sprouting [43] as well as frank neuronal loss [9], contribute significantly to the pathogenesis of HAND and other comorbid psychiatric complications associated with HIV. These post-mortem investigations have been supported by neuroimaging studies in patients with HIV that have also revealed cortical atrophy [44] and volumetric reductions in the caudate, amygdala and hippocampus [45–47] as well as diffusion tensor imaging (DTI) investigations demonstrating evidence of white matter abnormalities [48]. HIV neurotoxicity can be caused either by direct exposure of neurons to HIV and viral proteins or indirectly through the release of cytokines and chemokines by non-neuronal cells such as astrocytes and microglia [49]. In relation to HIV-associated neurotoxicity, a number of different HIV viral proteins have been shown to cause neurotoxicity, including gp120, gp41, Tat, Nef and Vpr [41, 50–55]. Evidence that HIV and viral proteins can cause neurotoxicity without the involvement of macrophages/microglia or astrocytes has come from some studies demonstrating direct toxicity of primary neurons following exposure to HIV proteins, in particular gp120 [56, 57]. However, given that neurons do not express CD4 and are therefore incapable of producing HIV infection, it is likely that the majority of HIV-associated neurotoxic effects are elicited through interaction with non-neuronal cells and subsequent release of soluble factors to cause neuronal injury. An example of this is increased release of TNF-α and IL-6 by macrophages, microglia and astrocytes following exposure to gp120 and Tat [58–60]. TNF-α release in turn causes further activation of microglia and macrophages as well as causing glutamatergic excitotoxicity and increasing BBB permeability [61], thus allowing increased trafficking of HIV-infected monocytes from the periphery. IL1-β, platelet-activating factor (PAF) as well as the free radical nitric oxide (NO) are also released by macrophages/microglia following exposure to gp120 and/or Tat [62]. TNF-α and IL1-β in turn cause release of the cytokine l-cysteine that interacts with N-methyl-d-aspartate (NMDA) to cause overactivity of these receptors and excess influx of calcium and glutamate, resulting in free radical formation and oxidative stress as well as excitotoxicity [63]. PAF released by neurons also act to increase glutamate release from presynaptic terminals [64]. In vitro experiments have shown that both PAF and TNF-α are capable of causing apoptosis and that compounds such as PAF acetylhydrolase that catabolize PAF are capable of preventing their combined effects [65], indicating a possible common mechanism or pathway for their pro-apoptotic effects. PAF-induced apoptosis of primary neurons has also been shown to be partially prevented by exposure to (S)-3,5-dihydroxyphenylglycine (DHPG), a group I metabotropic glutamate receptor (mGluR) agonist [64] and more recently by a novel tetrahydrofuran-derived bis-interacting ligand PMS777 that antagonizes the PAF receptor and attenuates PAF-induced neurotoxicity in SY5Y cells [66]. More recently, it has also been observed that release of a bone-marrow-derived cytokine, osteopontin, is increased in patients with HAD and that the levels are positively correlated with the degree of cognitive decline [67]. Osteopontin may therefore offer a new potential target for reducing HIV-associated CNS impairment.
HIV gp120
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree