Pediatric Epilepsy and Sleep Disorders
Paul R. Carney
James D. Geyer
Sachin Talathi
INTRODUCTION
Sleep disorders have an adverse impact on daily living for both children and their caregivers. Sleep disturbance and lack of restful sleep can masquerade as a myriad of clinical problems, including inattention, depression, headache, and seizures. Although most neurologic disorders are well characterized during the waking state, descriptions of pathophysiology, signs, and comorbidities are frequently poorly described during sleep. Physiologic changes associated with sleep can cause an alteration in signs and function during both rapid eye movement (REM) and nonrapid eye movement (NREM) sleep. These changes may include alterations in muscle tone, central control of autonomic functions, and changes in cortical neurotransmitter system interaction and balance. Epilepsy is also a disorder that affects every aspect of a child’s cognitive, social, and emotional well-being. When these disorders coexist, they can be both challenging to identify and differentiate and a burden to the young patient and their families.
This review is devoted to the relationships between epilepsy, sleep, and its disorders. The effects of epilepsy on sleep architecture and the quality of children’s sleep are reviewed. How sleep may affect epilepsy and the role of circadian rhythms is discussed next. Evidence regarding the relationship between epilepsy and both childhood sleep-related breathing disorders and restless legs syndrome (RLS) is summarized. Finally, a brief discussion highlighting differences between narcolepsy and epilepsy during childhood is provided. An outline of evaluation and treatment of the epileptic child with a sleep disorder ends this review.
PATHOPHYSIOLOGY OF SLEEP DISORDERS IN CHILDREN
Epilepsy and Sleep Architecture
Epilepsy has important effects on sleep and the sleep-wake cycle (1). Alterations in total sleep time, sleep latency, and spontaneous awakenings have been demonstrated in children (2). Epilepsy may affect both the quantity and the architecture of sleep. The effects of epilepsy on sleep vary depending on seizure type. In patients with primary generalized tonic-clonic seizures, the amount of REM sleep is decreased by 50% whereas in those suffering from secondarily generalized seizures, it may be as low as 41 % (3). Young children with infantile spasms and hypsarrhythmia and children with Lennox-Gastaut syndrome (4) also have decreased REM sleep. Prolonged sleep latency, an increase in the proportion of stages 1 and 2 NREM sleep, a decrease in the proportion of stages 3 and 4 NREM sleep, and an increase in the shifting between sleep stages have also been described. A decrease in the total amount of sleep in a 24-hour period is typical of children with infantile spasms and hypsarrhythmia (5). Patients affected with childhood absence epilepsy and epilepsy with myoclonic absences did not show such disturbances (6).
Epileptiform discharges themselves may affect sleep by causing disruption of normal sleep architecture. It might be expected, therefore, that in focal epilepsies where sleep disruption is less common, the stages of sleep would be close to normal. Indeed, it has been shown that among patients with complex partial seizures, only in those suffering from multiple nocturnal seizures is the proportion of REM sleep significantly lowered.
In patients with juvenile myoclonic epilepsy, the total NREM sleep duration was higher (46.7%) than that in age-matched controls (23%).
In patients with juvenile myoclonic epilepsy, the total NREM sleep duration was higher (46.7%) than that in age-matched controls (23%).
Not all forms of epilepsy have effects on sleep duration or architecture. It is interesting that in benign focal epilepsy, a disorder with no apparent impairment of mentation or symptoms of daytime dysfunction, despite nocturnal seizures, the pattern of sleep is largely unimpaired (7,8). Likewise, children with continuous slow spike waves of sleep have no associated sleep disorders.
Epilepsy Can Alter the Quality of Sleep
Children with epilepsy have more sleep problems than do their siblings or healthy controls. In addition, children with active seizure disorders have more complaints than do epileptic children who are seizure free. Children with idiopathic epilepsy had a greater incidence of parasomnias, bedtime difficulties, sleep fragmentation, and daytime drowsiness. Age is also a factor with younger children having more sleep problems than older children (9). Zaiwalla (10) and Stores found that parents describe their children’s sleep as “unrefreshing.” The unrefreshing quality was associated with frequent physiologic arousals, daytime sleepiness, and lethargy. They also found increased incidence of parasomnias and sleep fragmentation in these children. Rosen et al. (1) observed a correlation between nighttime awakening and daytime problems in learning and behavior in children with epilepsy. Stores (11) examined 79 children with epilepsy and 73 age-matched controls. Epileptic children aged 5 to 16 years were found to have more frequent sleep disturbances than matched controls. These disturbances included poor sleep quality and anxiety about sleeping. There was also a correlation between seizure frequency and anxiety about sleeping. In younger children between the ages of 5 and 11 years, poor sleep quality was associated with daytime inattention. This correlation was not noted in the older group. For instance, Hunt and Stores (12) found significant sleep disruption in younger children with tuberous sclerosis and active epilepsy. Problems with daytime inattention were seen more frequently in children who were experiencing frequent nocturnal seizures.
TABLE 32-1 ACTIVATION OF EEG DURING SLEEP | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||
Epilepsy Can Alter Circadian Rhythms
Fauteck et al. (13) have demonstrated lack of melatonin variation in children with epilepsy and poor sleep. They illustrate one example of restoration of normal melatonin levels in a child with a flat baseline melatonin curve, producing improvement in that child’s sleep and seizure control with 5 mg supplementation 1 hour prior to bedtime. Multiple studies have demonstrated improved sleep in developmentally disabled children with the use of melatonin (14).
Sleep Can Alter Epilepsy
Sleep tends to activate the electroencephalography (EEG) as delineated in Table 32-1. Interictal epileptiform activity in the EEG occurs more frequently during NREM sleep than during wakefulness, and tends to be suppressed by REM sleep (15). Representative samples of sleep are very important in the evaluation of the pediatric epilepsy patient. In a significant percentage of children, epileptiform activity appears in the EEG recording only while the patient sleeps. Conversely, epileptiform activity in the EEG recording is rarely isolated to wakefulness. In a study of 76 patients with epileptiform abnormalities on a sleep-deprived, awake, and asleep EEG recording, epileptiform activity was present in the wakeful portion in 9% of patients, in both the sleep and awake portions, in 51%, while in the sleep-only portion, it was present in 40% of patients (16). Sleep deprivation is commonly used to increase the likelihood of obtaining a significant EEG recording in children. However, it is unclear whether this is best achieved by natural sleep, sedated sleep, or sleep deprivation (17). The existing literature supports a role for sleep deprivation, separate from the induction of sleep with a hypnotic agent, in activating epileptiform discharges (17). A sleep-deprived patient is more likely to provide an adequate sleep tracking with activation of epileptiform discharges. Interestingly, it has been shown that sleep deprivation can increase the occurrence of epileptiform abnormalities in the wake portion of the EEG in a child who previously had a normal awake EEG (18).
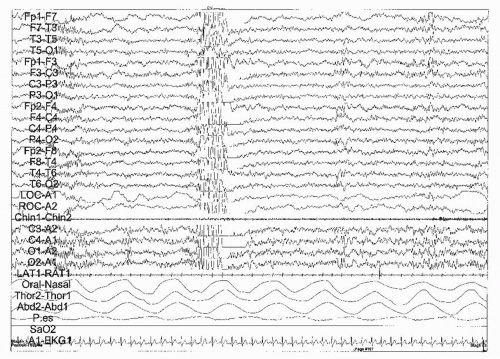
Certain EEG characteristics describe the different stages of REM and NREM sleep and are significant in the diagnosis of the different types of epilepsy. For instance, in childhood absence epilepsy, generalized spike-and-wave discharges are activated by NREM sleep, especially during
the first sleep cycle (19) (Fig. 32-1). The rate and shape of the epileptiform activity are altered by sleep. While in REM sleep, the spike and wave discharges are shorter and less frequent; they are also shorter but more frequent during stage 1 NREM sleep. Discharges become more irregular and polyspikes may appear during stage 2 NREM sleep. Finally during stages 3 and 4 sleep, the rate of discharges becomes less rhythmical and may be reduced to <2 Hz.
the first sleep cycle (19) (Fig. 32-1). The rate and shape of the epileptiform activity are altered by sleep. While in REM sleep, the spike and wave discharges are shorter and less frequent; they are also shorter but more frequent during stage 1 NREM sleep. Discharges become more irregular and polyspikes may appear during stage 2 NREM sleep. Finally during stages 3 and 4 sleep, the rate of discharges becomes less rhythmical and may be reduced to <2 Hz.
In juvenile myoclonic epilepsy the typical polyspikes are increased during sleep onset and especially during nocturnal arousals, but usually disappear during both REM and NREM sleep (19). Gigli et al. (21) developed the term “cyclic alternating pattern” (CAP) for the degree of EEG activation during NREM sleep in patients with juvenile myoclonic epilepsy. They reported that the ratio between total CAP duration and total NREM sleep duration was significantly higher in these patients (46.7%) than in age-matched controls (23%). Strong activation of epileptiform EEG abnormalities during one of the two phases of CAP, with strong inhibition in the other phase, was also reported. Children with infantile spasms produce an awake EEG tracing that often consists of hypsarrhythmia (West syndrome). NREM sleep can alter this pattern, such that bursts of more diffuse polyspike waves and more synchronous slow spike and waves are observed. Spindles can be seen, superimposed on a generalized low-amplitude tracing, in between these bursts. These periods of bursts and low amplitude can alternate, resembling burst suppression pattern (19). The hypsarrhythmic pattern may be completely suppressed by REM sleep, normalizing the EEG. Recently, Kohyama et al. (22) report that in children with infantile spasms, seizure control could be predicted by an increase in the number of spontaneous horizontal eye movements associated with phasic chin muscle activity during REM sleep. Those patients with a good response to valproate, clonazepam, and/or zonisamide had significantly fewer simultaneous phasic events than did those poor responders who required hormonal therapy (22). In those patients with good response to treatment, REM phasic activity was similar to that in controls. They postulate that an increase in REM phasic activity suggests loss of inhibition from the pons to the motor neurons controlling REM muscle atonia. During wakefulness, children with Lennox-Gastaut syndrome show a typical 2- to 2.5-Hz slow spike and wave pattern (Fig. 32-2). However, during NREM sleep, frontally dominant bilateral rhythmic discharges with a frequency
of about 10 Hz occur, which are considered to be an essential feature of this syndrome, and which are sometimes but not always accompanied by tonic seizures. NREM sleep characteristically increases the frequency of interictal spikes in those patients with partial seizures, which may be idiopathic cryptogenic or symptomatic and is seen most prominently in benign partial epilepsy with centrotemporal spikes (rolandic epilepsy) (19).
of about 10 Hz occur, which are considered to be an essential feature of this syndrome, and which are sometimes but not always accompanied by tonic seizures. NREM sleep characteristically increases the frequency of interictal spikes in those patients with partial seizures, which may be idiopathic cryptogenic or symptomatic and is seen most prominently in benign partial epilepsy with centrotemporal spikes (rolandic epilepsy) (19).
Myoclonic and generalized tonic-clonic seizures occur upon awakening, not just in the morning but also after naps and as the patient falls asleep (21) (Fig. 32-3). Seizures can also be induced by relative sleep deprivation in those patients with temporal lobe epilepsy (23). Lanz studied the relationship between sleep and the occurrence of generalized tonic-clonic seizures in 2,110 patients. He found that in 45% of the patients seizures occurred concomitantly with sleep, showing two peaks, the first near bedtime, between 21.00 and 23.00 hours; the second, much later, between 03.00 and 05.00 hours. Absence seizures, which, interestingly, are more common in the evening and upon awakening, also show an association with sleep, in the form of eye fluttering, which correlates with EEG findings, including discharges of 3-Hz spike and wave complexes (24) (Fig. 32-1). Infantile spasms also occur more frequently in the period that precedes or just follows from sleep, whereas they rarely occur during NREM sleep and never during REM. The brief, generalized tonic seizures associated with Lennox-Gastaut syndrome occur more frequently not only in clusters upon awakening but also during NREM sleep (25) (Fig. 32-3).
Some types of seizures are predominantly nocturnal and only rarely occur during the daytime. In patients with myoclonic-astatic epilepsy (Doose syndrome), tonic seizures, which present with 10- to 15-Hz spike series, occur almost exclusively during sleep (26). Other seizures that occur mainly during sleep include benign partial epilepsy with centrotemporal spikes (rolandic epilepsy) and complex partial seizures of frontal lobe origin. The latter consists of multiple short seizures during sleep, particularly during NREM sleep. Seventy-five percent of children with rolandic epilepsy have seizures exclusively during either daytime or nighttime sleep, approximately 15% have seizures whether they are awake or asleep, and 10% to 20% experience seizures only when awake. Rolandic seizures
are more likely to occur not only during sleep but when they are nocturnal, they are more likely to be longer, and are more likely to become secondarily generalized (19). Epilepsies with seizures occurring primarily during the day are rare and can be seen in young patients with benign epilepsy of childhood with occipital paroxysms.
are more likely to occur not only during sleep but when they are nocturnal, they are more likely to be longer, and are more likely to become secondarily generalized (19). Epilepsies with seizures occurring primarily during the day are rare and can be seen in young patients with benign epilepsy of childhood with occipital paroxysms.
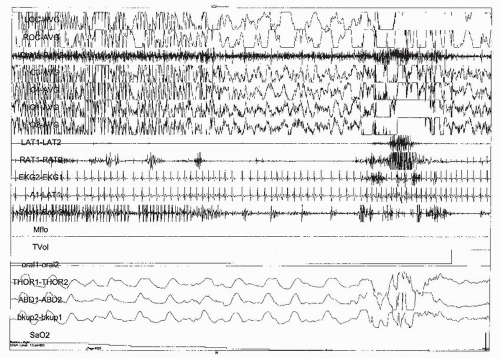 FIGURE 32-3 Polysomnogram: CPAP montage; 30-s page. Clinical: 17-year-old boy with epilepsy and obstructive sleep apnea. Staging: unable to accurately stage because of generalized spike and wave discharges during this generalized tonic-cloni c seizure and subsequent postictal slowing. Respiratory: ictal and postictal obstructive apnea associated with an arousal and an oxygen desaturation. EEG: generalized spike and wave discharges. At the end of the seizure (*) there is generalized delta activity during the postictal phase. Artifact: the tidal volume channel has artifact caused by the mask being pulled from the patient’s face during postictal confusion. (From Geyer JD, Payne TA, Carney PR, et al. Atlas of digital polysomnography. Philadelphia, PA: Lippincott Williams & Wilkins, 2000:208, with permission.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|