Pediatric Ischemic Stroke and Arteriopathy
Key Points
The spectrum of transient or progressive cerebral arteriopathy of childhood may not be familiar to those with a predominantly adult-based practice.
Intracranial dissection is a potentially fatal condition in the differential diagnosis for childhood stroke that can mimic angiographically the more benign condition of focal cerebral arteriopathy (FCA).
Pediatric stroke, ischemic or hemorrhagic, is no longer considered to be a rare disease with a recognized incidence of 2 to 6/100,000 (1). The risk factors and epidemiology of pediatric stroke differ from those of the adult population, with some particular phenomena evident on imaging that have substantial bearing on the likely prognosis and risk of recurrence. Furthermore, pediatric stroke has well-recognized associations with several syndromic disorders that have other imaging features that warrant specific evaluation during noninvasive and angiographic imaging.
Perinatal Stroke
Pediatric stroke is considered in two time periods, perinatal and late childhood. Perinatal stroke occurs between 20 weeks of fetal life and the 28th postnatal day, and has an incidence of about 1/4,000 births (1,2). Risk factors include any causes of chorioamnionitis, premature membrane rupture, cardiac anomalies, and birth asphyxia (3,4). Venous sinus compression and thrombosis have an association with maternal or fetal factor V Leiden, antiphospholipid antibody activity, and protein C deficiency. Arteriopathy is considered rare in this subgroup, and catheter angiographic evaluation is rarely warranted.
Childhood Stroke
The risk factors for childhood stroke demonstrate significant genetic and geographic variabilities, and broadly speaking include trauma, infection, arteriopathy (abnormal looking vessels of any cause), cardiac anomalies, prothrombotic conditions, and rarer genetic conditions including Menke’s disease, Fabry’s disease, and incontinentia pigmenti (4). Stroke accounts for approximately 10% of childhood mortality in some series, and has substantial impacts on cognitive and psychomotor development, particularly if it is recurrent. Childhood strokes also have a greater propensity to present with seizure than is the case with adults.
Congenital cardiac anomalies account for between 20% and 40% of childhood strokes (5). The remaining 60% to 80% demonstrate an abnormal appearance of the intracranial or extracranial vessels, generically termed “arteriopathy” (6,7). It is in the parsing of the latter group that imaging and angiography can play an important role.
Pediatric Cerebral Arteriopathy
In most series, some form of arteriopathy will be demonstrated in the dominant majority (80%) of pediatric patients with childhood stroke. The most commonly recognized entities are Focal Cerebral Arteriopathy of Childhood (also termed Transient Cerebral Arteriopathy) (8,9), arterial dissection, primary Moya-Moya disease particularly in Asian populations in whom it can account for a majority of cases of arteriopathy, and Moya-Moya syndrome secondary to other entities discussed below.
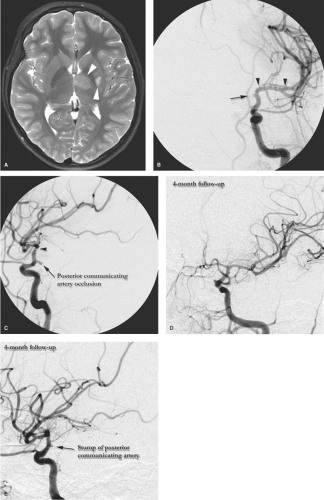
Focal Cerebral Arteriopathy (FCA) accounts for approximately half of all stroke related to arterial abnormalities in the pediatric group, and is itself a heterogeneous group (Fig. 22-1). Most cases are ascribed to an immunologic reaction to antecedent varicella infection (10,11,12), or other infections such as Lyme disease or enterovirus (13). FCA is defined as a unilateral, nonprogressive, somewhat localized vasculopathy, usually confined to the region of the carotid terminus and its major branches. It most commonly presents with ischemic complications of lenticulostriate compromise, or ipsilateral hemispheric watershed infarctions. It generally runs its course in 1 to 3 months and most patients (75%) recover well (14). Due to its presumed immunologic basis, it may represent a transient or localized form of primary angiitis of the central nervous system, although this is debated. Focal or transient arteriopathy is discerned from the 25% of patients who demonstrated signs at presentation or develop subsequent more diffuse findings suggesting the presence of Progressive Arteriopathy of Childhood. Progressive arteriopathy is associated with a worse prognosis and outcome, due to a recurrent and worsening clinical pattern of strokes.
Sometimes it is not possible on the first angiogram to be specific in the diagnosis of FCA, and a second study at a second time point after 6 months may be necessary. Early Moya-Moya disease may present initially with unilateral findings. Particular caution is warranted not to overlook the possibility of intracranial dissection which can easily mimic and imaging findings of FCA.
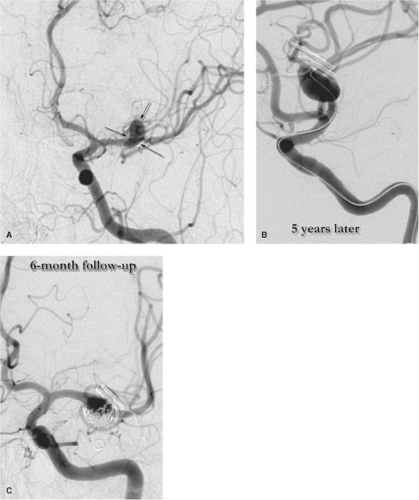
Intracranial Dissection is a well-recognized disease of childhood, accounting for approximately 10% of non-cardiac–related strokes (Figs. 22-2). It seems to have a higher association in younger children with trauma, including child abuse, whereas in older children, it may be seen following normal effortful behavior, similar to the presentation in young adults. It seems questionable to include dissection with the category of nonprogressive arteriopathy, but it is likely that most dissections heal spontaneously. Some, however, can present with or progress to intracranial occlusions or rupture with pseudoaneurysm formation. Complicated intracranial dissection tends to have a poor prognosis and can be initially diagnosed as more benign FCA (15).
Progressive Cerebral Arteriopathy of Childhood encompasses the 25% of pediatric stroke patients who have disease more extensive than FCA and who generally tend to have a high likelihood of stroke recurrence and neurologic impairment. Chief among these entities are primary Moya-Moya disease, and secondary Moya-Moya syndromes due to sickle cell disease (16,17), previous brain irradiation (18), neurofibromatosis type 1 (4), neurofibromatosis type 2, hemolytic–uremic syndrome, Down syndrome (19,20,21), and even rarer entities such as PHACES (posterior fossa abnormalities,
hemangiomas, arterial anomalies, cardiac anomalies, coarctation, eye abnormalities, sternal defects) (22), Robinow syndrome (23), and Seckel syndrome (24,25).
hemangiomas, arterial anomalies, cardiac anomalies, coarctation, eye abnormalities, sternal defects) (22), Robinow syndrome (23), and Seckel syndrome (24,25).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree