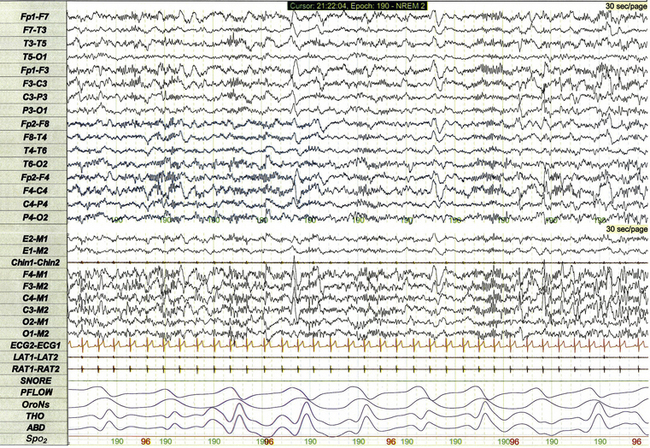
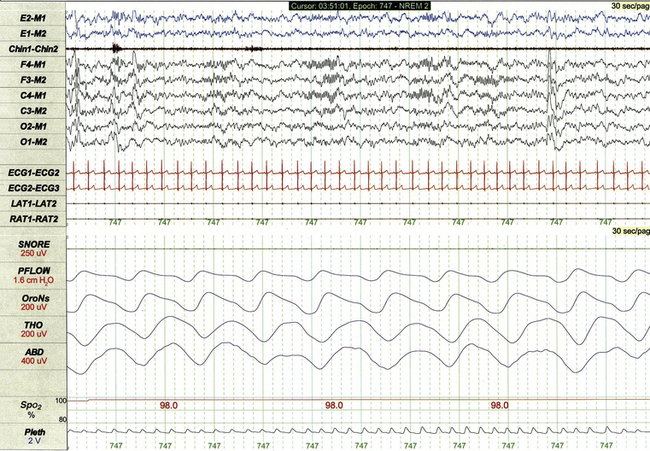
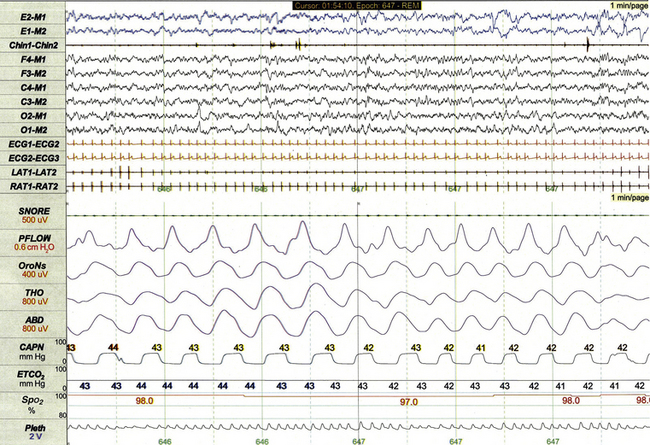
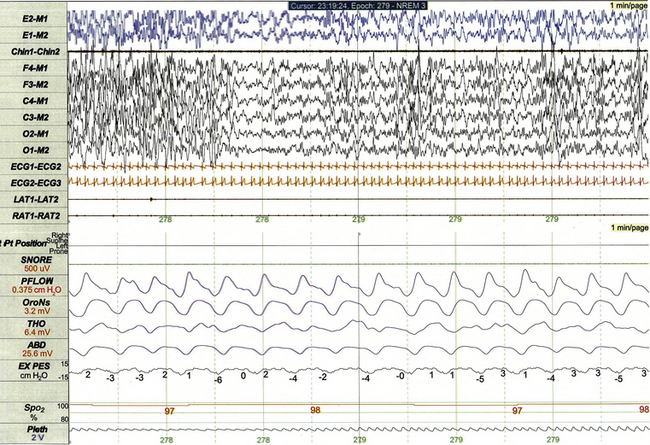
Chapter 17 Figure 17.1 demonstrates a standard recording montage for children that conforms to the recommended specifications of the American Academy of Sleep Medicine (AASM) Manual for the Scoring of Sleep and Associated Events. Electroencephalography (EEG) recording includes frontal (F4, F3), central (C4, C3), and occipital (O2, O1) leads referenced to the contralateral mastoid (M1, M2). Bilateral electro-oculogram (EOG) leads (E2, E1) permit detection of the slow eye movements of light sleep and the rapid movements associated with stage R. Surface electromyography (EMG) over the chin (Chin1-Chin2) permits detection of increased muscle tone with arousals or atonia associated with stage R sleep. Additional EMG leads over the left and right anterior tibialis muscles (LAT1-LAT2, RAT1-RAT2) permit recording of periodic limb movements and other limb movements associated with arousals or sleep-related movement disorders. Electrocardiogram (ECG) channels provide concurrent data reflecting cardiac rate and rhythm. FIGURE 17.1 Standard pediatric recording montage demonstrating stage N2 sleep in a healthy 5-year-old (30-second epoch). The PSG recording montage for children can be customized depending on the nature of the sleep disorder being investigated. End-tidal carbon dioxide (ETCO2) or transcutaneous carbon dioxide (TcCO2) monitoring (Fig. 17.2) during PSG is appropriate for children with neuromuscular disorders, morbid obesity, Chiari I malformation, and other medical conditions associated with increased risk for nocturnal hypoventilation. FIGURE 17.2 Pediatric polysomnographic montage with supplemental end-tidal CO2 (ETCO2) monitoring demonstrating normal CO2 levels during stage R sleep in a 9-year-old boy (1-minute epoch). ETCO2 is calculated by measuring peak CO2 levels at the end of each breath as displayed by the capnogram (CAPN). The channel labeled ETCO2 displays a rolling average of the peak levels recorded on the capnogram. Use of Pes monitoring improves the sensitivity of PSG for detection of chronic partial airway obstruction and other findings associated with upper airway resistance syndrome (UARS). The technique uses a small water- or air-filled esophageal catheter attached to a pressure transducer for direct quantitative assessment of Pes fluctuations during each respiratory cycle (Fig. 17.3). Although minimally invasive, Pes monitoring can detect varieties of sleep-related airway obstruction that are often not evident on standard PSG. The technique is well tolerated by most school-age children and has negligible impact on sleep architecture. FIGURE 17.3 Pediatric polysomnographic montage with supplemental esophageal pressure (Pes) monitoring demonstrating normal Pes fluctuations during stage N3 sleep in a 10-year-old boy (1-minute epoch). The esophageal pressure tracing (labeled EX PES) demonstrates normal fluctuations (0 to −10 cm H2O, measured from peak to trough) with inspiration. Sixteen-lead EEG can be performed during pediatric PSG (Fig. 17.4) when sleep-related seizures represent a clinical consideration. Technically satisfactory video recording is particularly important during expanded EEG studies to verify the presence or absence of any clinical symptoms coinciding with EEG findings. Although technically straightforward to perform, interpretation of expanded EEG studies is time consuming and requires that the polysomnographer be experienced in the interpretation of normal and abnormal EEG findings for the pediatric age-group. Infants often enter sleep with a period of stage R sleep, which constitutes almost half of total sleep time in term infants (Fig. 17.5). In Figure 17.6 the EEG background during stage R sleep for this 2-month-old infant is characterized by mixed frequencies of somewhat higher amplitude than those typical of REM sleep for older children. The rapid eye movements demonstrated in this epoch are not always apparent in younger infants, whereas the reduced EMG activity and irregular respiration demonstrated represent somewhat more consistent findings. FIGURE 17.5 Hypnogram illustrating sleep cycling for a 5-month-old boy. FIGURE 17.6 Infant rapid eye movement (REM) sleep (active sleep, stage R). Stage N sleep during infancy is characterized primarily by high-amplitude delta and theta frequencies associated with deep, regular respiration and less-frequent limb and body movements compared to infant REM sleep (Fig. 17.7, demonstrating findings for the same 2-month-old infant shown in Fig. 17.6). FIGURE 17.7 Infant non–rapid eye movement sleep (quiet sleep, stage N). Periodic breathing is a respiratory pattern characterized by cyclical periods of normal breathing interrupted at regular intervals by brief pauses in respiration (Fig. 17.8). This pattern is observed most commonly in premature infants but is occasionally observed in younger term infants as well. Scoring rules established by the 2007 AASM Manual for the Scoring of Sleep and Associated Events specify that periodic breathing may be scored in the presence of more than three episodes of central apnea separated by no more than 20 seconds of normal breathing. FIGURE 17.8 Periodic breathing.
Pediatric Polysomnography
Standard and Custom Polysomnographic Montages
Normal Sleep in Infants

Typical characteristics of infant sleep include a sleep-onset rapid eye movement period, a high proportion of stage R sleep (49.8% in this study), and shorter sleep cycling time than the 90 minutes typical for older children.
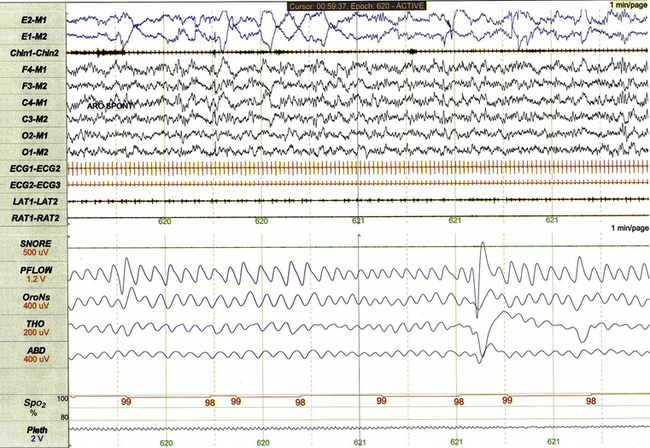
This 1-minute polysomnographic epoch for a 2-month-old girl with Prader-Willi syndrome demonstrates clearly evident rapid eye movements, muscle atonia with phasic twitches on chin electromyogram, and mixed-frequency electroencephalographic background. The irregular respiratory rate and effort in this epoch are also typical for infant REM sleep.
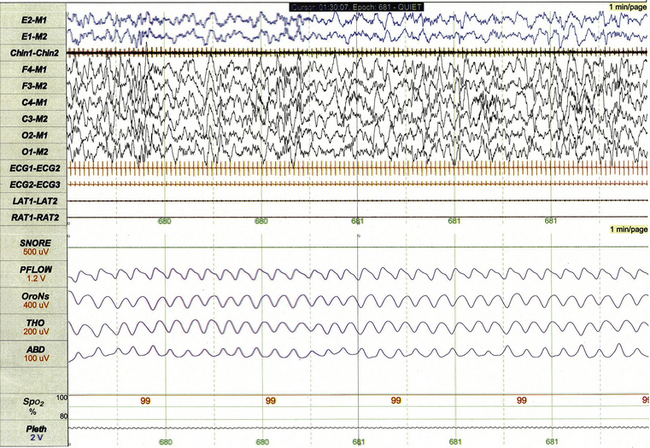
This 1-minute polysomnographic epoch for a 2-month-old-girl with Prader-Willi syndrome demonstrates typical findings of quiet sleep, including higher baseline chin electromyographic tone compared to active sleep, deep and regular respiration, and high-amplitude delta and theta frequency electroencephalographic (EEG) background. Immature sleep spindles are apparent in the central EEG channels.
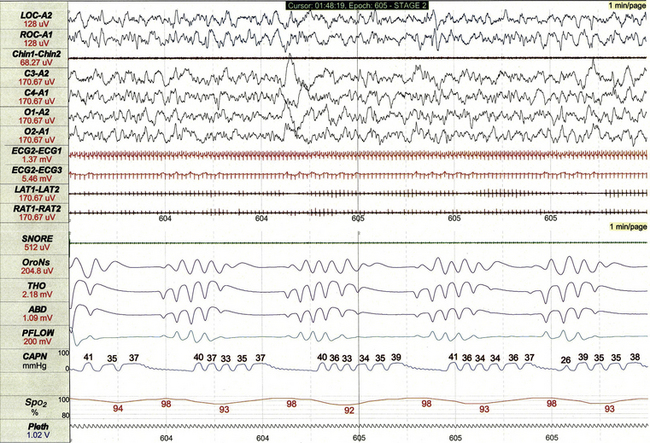
This 1-minute polysomnographic epoch demonstrates periodic breathing in an otherwise healthy 2-month-old infant born at term. Although the central apneas are associated with desaturations of SpO2 of 5% to 6% below baseline, nadir levels of SpO2 are only 92% to 94%. Note that flow on the capnogram (CAPN) channel is delayed compared to other effort and airflow channels, reflecting the cannula transit time of exhaled air before CO2 levels can be measured.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree