Chapter 49 Percutaneous and Minimally Invasive Approaches to Decompression and Arthrodesis of the Cervical Spine
Dorsal Minimally Invasive Approaches for the Cervical Spine
Dorsal decompressive procedures are fundamental tools in the surgical treatment of symptomatic cervical degenerative spine disease.1–4 Even as ventral cervical procedures have gained prominence, posterior cervical laminoforaminotomy still provides symptomatic relief in 92% to 97% of patients with radiculopathy from foraminal stenosis or lateral herniated discs.3,5 Similarly, dorsal cervical decompression for cervical stenosis achieves neurologic improvement in 62.5% to 83% of myelopathic patients undergoing either laminectomy or laminoplasty.4,6–8 Moreover, these operations avoid the complications attendant to ventral approaches to the cervical spine, namely, esophageal injury, vascular injury, recurrent laryngeal nerve paralysis, dysphagia, and accelerated degeneration of adjacent motion segments after fusion.9–11
However, open dorsal approaches to the cervical spine require extensive subperiosteal stripping of the paraspinal musculature that leads to postoperative pain, spasm, and dysfunction and can be persistently disabling in 18% to 60% of patients.4,9,12,13 Furthermore, preoperative loss of lordosis and long segment decompressions increase the risk for postoperative sagittal plane deformity,14–17 a complication that frequently prompts instrumented arthrodesis at the time of laminectomy. Employing these extensive posterior fusion techniques increases operative risks, time, and blood loss; exacerbates early postoperative pain; and potentially contributes to adjacent-level degeneration.
The fundamental tenet of minimal access techniques is reduction of approach-related morbidity. To that end, the advent of muscle-splitting tubular retractor systems and the use of endoscopic technology or the microscope have allowed for the application of minimally invasive techniques to dorsal cervical decompressive procedures13,18–35 and fixation.36–40
Spurling, Scoville, and Frykholm were the first to describe the open cervical foraminal decompression between 1944 and 1947.41–43 In 1983 Williams reported the first microsurgical technique for dorsal cervical foraminotomy.44 Several minimally invasive dorsal cervical techniques were described after that.18–40 To avoid confusion, and to simplify the description of all these techniques, we divided them into two main approaches: (1) the minimally invasive midline cervical approach, and (2) the minimally invasive paramedian (transtubular or transmuscular) cervical approach. An endoscope or microscope could be used in either approach. These approaches are used to perform laminotomy/foraminotomy/discectomy, laminectomy, laminoplasty,34,35 and lateral mass fixation.36–40
Indications
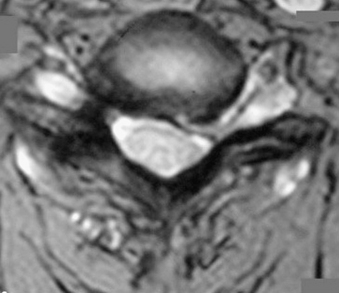
The operative indications for minimally invasive laminotomy/foraminotomy/discectomy are (1) unilateral single-root (Fig. 49-1) or multiple-root cervical radiculopathy from lateral disc herniations or foraminal stenosis (single-level or multilevel), without instability, significant kyphosis, or severe axial neck pain; (2) persistent or recurrent root symptoms following anterior cervical discectomy and fusion; (3) cervical disc disease in patients for whom anterior approaches are relatively contraindicated (e.g., ventral neck infection, tracheostomy, prior irradiation); and (4) cervicothoracic disc herniation and radiculopathy, to avoid ventral approach potential complications and when the ventral approach is not feasible (short neck or others). A ventral approach should be considered in case of same-level bilateral radiculopathy, central disc herniation, significant kyphosis, and severe axial neck pain.
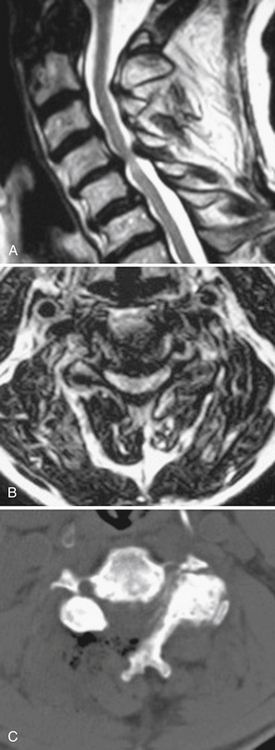
Most patients who are candidates for a noninstrumented, dorsal cervical decompression are also candidates for a minimally invasive cervical decompression. These are selected patients with clinical myelopathy or myeloradiculopathy, radiographic evidence of spinal cord compression from one to three adjacent cervical levels, and a lordotic cervical spine (Fig. 49-2). Contraindications include loss of the normal cervical lordosis, severe ventral disease (disease that extends for more than three levels), and segmental instability.
Minimally invasive dorsal cervical fixation could be applied in case of facet dislocation or segmental instability or to support ventral instrumentation. Few clinical reports are available in the literature.36,37,39 Minimally invasive cervical laminoplasty is still undergoing investigation.
Operative Setup
General endotracheal anesthesia is induced on a standard electric operating table. A neurophysiologic monitoring array with capabilities for somatosensory evoked potentials (SSEPs), motor evoked potentials (MEPs), and free running electromyography (EMG) is put in place. In cases of myelopathy, a fiberoptic intubation may be elected and evoked potentials are compared before and after positioning to identify positioning-related cord ischemia. Maintenance of normotension to avoid spinal cord hypoperfusion is best directed with continuous blood pressure measurements afforded by an arterial line. Measures to detect and treat air embolism, such as a precordial Doppler and a central line, are options but have not yet proven necessary. Given the small exposure, the risk of air embolism is low. A urinary catheter is generally not necessary for one- or two-level procedures. Routine perioperative antibiotics are administered. Relaxants are minimized after induction to allow for effective neurophysiologic monitoring.
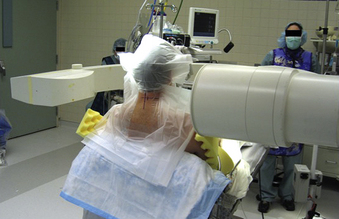
Posterior cervical approaches might be performed with the patient in the prone or sitting position. With the prone position, the head is held with a Mayfield pin-holder or a well-padded horseshoe-shaped headrest, with slight flexion. The operating table is tilted in a reverse Trendelenburg position to ensure that the cervical spine is parallel to the floor. The senior author (RGF) prefers the sitting position (Fig. 49-3) because it confers advantages of decreased epidural bleeding, decreased pooling of blood in the operative field, decreased anesthesia time, and gravity-dependent positioning of the shoulders for better lateral fluoroscopic images. The table is turned 180 degrees relative to the anesthesiologist. The patient’s head is fixed in a Mayfield head holder. The table is manipulated to place the patient in a semisitting position with the head flexed and the neck straight and perpendicular to the floor.
Paramedian Approach
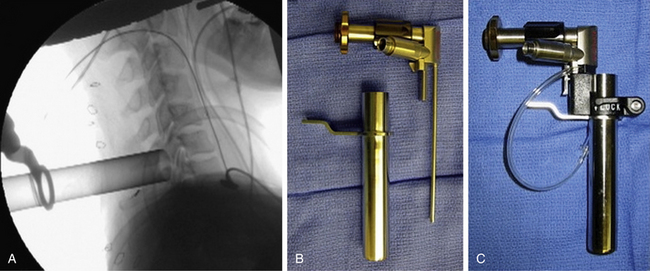
The operative level(s) and entry point are confirmed on lateral fluoroscopy with a K-wire. A 1.8-cm longitudinal incision is marked out approximately 1.5 cm off the midline on the operative side and injected with local anesthetic. For two-level procedures the incision should be placed midway between the targeted levels. After an initial stab incision, the K-wire is advanced slowly though the subcutaneous tissue and docked on the cervicodorsal fascia. Once an optimal trajectory is established, the fascia is incised with a scalpel to accommodate dilators. The fascia is retracted, and the smallest dilator is placed through the posterior cervical musculature under fluoroscopic guidance and docked at the lamina-facet junction of the level of interest. A slightly lateral trajectory is advised to avoid the spinal canal and ensure contact with the lateral mass. Successive tubular muscle dilators are carefully and gently inserted, remembering that the axial forces that are routinely applied during muscle dilation in the lumbar spine are hazardous in the cervical spine. After dilation, the final tubular retractor is placed and secured over the junction of the lamina and the facet with a table-mounted flexible retractor arm and the dilators are removed. The following steps are performed under microscopic magnification or using loupes or an endoscope. The endoscope is inserted and attached to the tubular retractor (Fig. 49-4). Monopolar cautery and pituitary rongeurs are used to clear the remaining soft tissue off of the lateral mass and lamina of interest, taking care to start the dissection over solid bone laterally.
Laminotomy/Foraminotomy/Discectomy
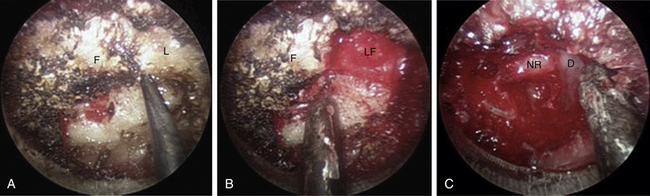
The medial facet/interlaminar space junction is identified. Using a high-speed drill, a partial laminotomy-facetectomy is performed beginning at the medial facet/interlaminar space and going laterally, without exceeding 50% facet removal, to maintain biomechanical integrity. The dorsolateral portion of the superior lamina and the medial part of the inferior articular facet are removed first. This will permit the removal of the lateral corner of the inferior lamina and the medial part of the superior articular facet, exposing the medial border of the caudal pedicle. The nerve root is located directly above the caudal pedicle and anterior to the superior articular facet. The ligamentum flavum can be removed medially after the foraminotomy to expose the lateral edge of the dura and proximal portion of the nerve root. Progressive lateral dissection can then proceed along the root as it enters the foramen. The venous plexus overlying the nerve root should be carefully coagulated with bipolar cautery and incised. With the root well visualized, a fine-angled dissector can be used to palpate ventrally to the nerve root for osteophytes or disc fragments. Should an osteophyte be present, a down-angled curette may be used to tamp the material further ventrally into the disc space or fragment it for subsequent removal. In the case of a soft disc herniation, a nerve hook may be passed ventrally and inferiorly to the root to gently tease the fragment away from the nerve for ultimate removal with a pituitary rongeur. In either case, additional drilling of the superomedial quadrant of the caudal pedicle allows greater access to the ventral pathology and obviates the need for excessive nerve root retraction superiorly (Fig. 49-5).
Decompression for Stenosis
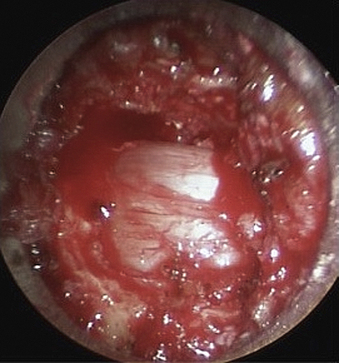
In this case, ipsilateral laminotomy of the levels of interest is performed and the ligamentum flavum is left in place to protect the dura. The tube is then angled about 45 degrees off the midline such that the tube is oriented to visualize the contralateral side. A plane between the ligament and undersurface of the spinous process is gently dissected with a fine curette. The drill with guard sleeve extended is then used to progressively drill the undersurface of the spinous process and contralateral lamina all the way to the contralateral facet. This initial decompression allows greater working space within which to remove hypertrophied ligament while avoiding downward pressure on the dura and spinal cord. Dissection and removal of the ligament with curettes and Kerrison rongeurs may now proceed safely. Any compressive elements of the contralateral facet or the superior edge of the caudal lamina may also be drilled off or removed with Kerrison rongeurs at this time because their impact on the dura is more apparent with the ligament removed. After gently confirming decompression over to the contralateral foramen with a fine probe, the tube is returned to its original position to complete the ipsilateral removal of ligament and bone. This should then reveal completely decompressed and pulsatile dura (Fig. 49-6). If indicated, ipsilateral foraminotomy, as described earlier, also may be performed at this time.
Lateral Mass Fixation
After exposure of the facet joint, a hand drill is used to create a pilot hole. The starting point is 1 mm medial to the midpoint of the lateral mass, and the trajectory will be 25 degrees lateral and parallel to the facet joint. Appropriate dimension screws are inserted after taping, and a rod is fixed with set screws. A midline incision is preferred in this case for the transmuscular approach. The same incision could be used for both sides. Also, fluoroscopic guidance is preferred.
Outcomes and Results
Favorable outcomes were reported in the literature for posterior cervical foraminotomy with a range between 75% and 100%.1,3,9,44–48 Krupp et al. separated the outcomes by soft, hard, and mixed pathology, with favorable outcomes of 98%, 84%, and 91%, respectively.45 Jodicke et al. reported a significantly better outcome for soft discs compared to hard discs in early follow-up, but no difference was found at long-term follow-up.47
The reports of minimally invasive, microscopic, and microendoscopic posterior cervical formainotomy have demonstrated equivalent efficacy to the open technique, but the blood loss, length of stay, and postoperative pain medication usage were reduced with the minimally invasive techniques.9,13,20,21,25,26,48 The senior author and Khoo prospectively used cervical microendoscopic posterior foraminotomy in 25 patients and compared the results with another 26 patients treated via open cervical laminoforaminotomy.9 The microendoscopic group had a lower overall operative time (115 vs. 171 minutes), less blood loss (138 vs. 246 mL), shorter postoperative hospital stay (20 vs. 68 hours), and fewer postoperative narcotic medications (11 vs. 40 equivalents) when compared with the open technique group.
Ruetten et al. conducted a prospective, randomized, controlled study with lateral cervical herniations, operated either in a full endoscopic posterior foraminotomy (89 patients) or conventional microsurgical anterior technique with fusion/plating (86 patients), with 2 years of follow-up.20 There was no significant difference between the groups in the clinical outcome, revision, or complication rates. Preservation of motion was conserved in the full endoscopic posterior group.
Perez-Cruet and the senior author have reported on five patients undergoing cervical microendoscopic decompression for stenosis at one, two, or three levels.16 All patients demonstrated improvement in their myelopathy and returned to work with the only complication being one unintended durotomy that sealed spontaneously. Yabuki et al. performed endoscopic partial laminectomy in 10 patients with degenerative cervical compressive myelopathy.28 All patients experienced symptomatic improvement with slight postoperative wound pain. The mean operative duration was 164 ± 35 minutes and the intraoperative blood loss was 45.5 ± 27 mL. Boehm et al. reported the results of 13 patients who were operated on under the microscope through a transmuscular tubular approach.30 Nine of these patients suffered from cervical myelopathy. The average follow-up was 17 months. The neck disability index score and visual analogue scale score were significantly improved. Thirty-three percent showed complete recovery of the preoperative neurologic deficit, while 67% had improvement.
Wang et al. reviewed retrospectively 18 patients treated with lateral mass screws placed in a minimally invasive fashion.37 In two cases, the minimally invasive technique was converted to the standard open technique because of inability to visualize anatomic landmarks on fluoroscopy (bulky shoulders). Successful fusion was documented in all cases, and there were no hardware failures during the minimum 2 years of follow-up. Two patients were lost to follow-up after 6 months.
Complications
The posterior cervical foraminotomy is a safe procedure associated with a low rate of complications (1–15%),1,3,9,13,20,21,25,26,44–48 with wound infection and dural tear contributing to the majority of them. The senior author has no infection to date in his microendoscopic series, and the unintended durotomy rate has dropped from 8% in the initial series of patients9 to around 1% more recently. Direct suture repair of durotomy is difficult through the narrow-diameter tubes or small incisions. Therefore one technique for handling small defects is to simply cover the durotomy with muscle, fat, hemostatic gelatin (Gelfoam, Baxter Healthcare, Glendale, CA), or dural susbstitute followed by fibrin glue or synthetic sealant such as Coseal (Baxter Healthcare, Glendale, CA). Using this approach, overnight bedrest is usually sufficient to seal the defect. For larger dural tears that cannot be primarily closed, 2 to 3 days of lumbar cerebrospinal fluid (CSF) drainage may prevent a leak. Ultimately, the small opening and relative lack of dead space after minimally invasive procedures have made the incidence of postoperative pseudomeningoceles and CSF49-cutaneous fistulae negligible.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree