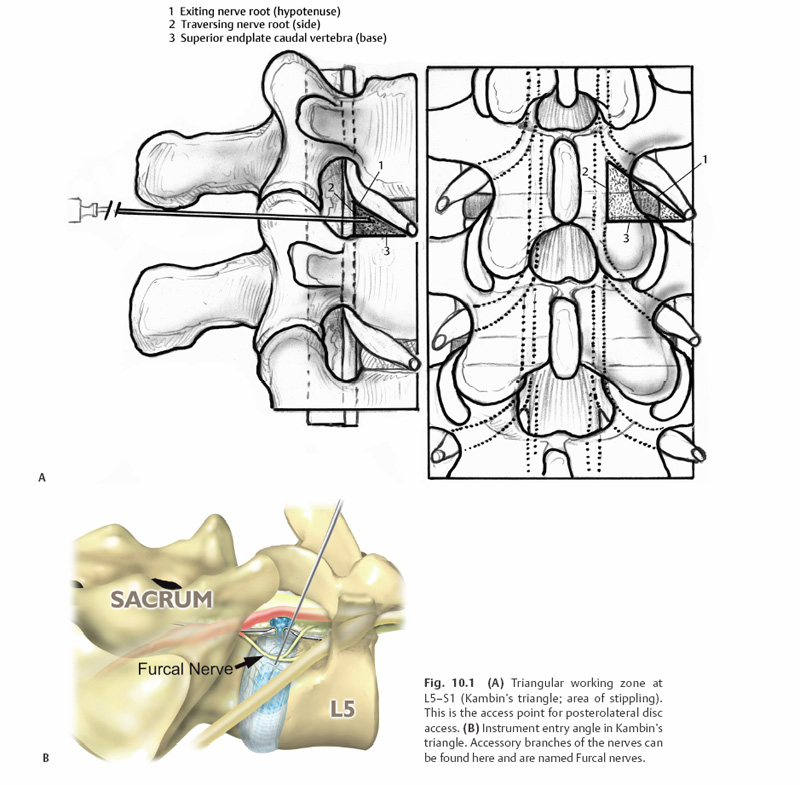
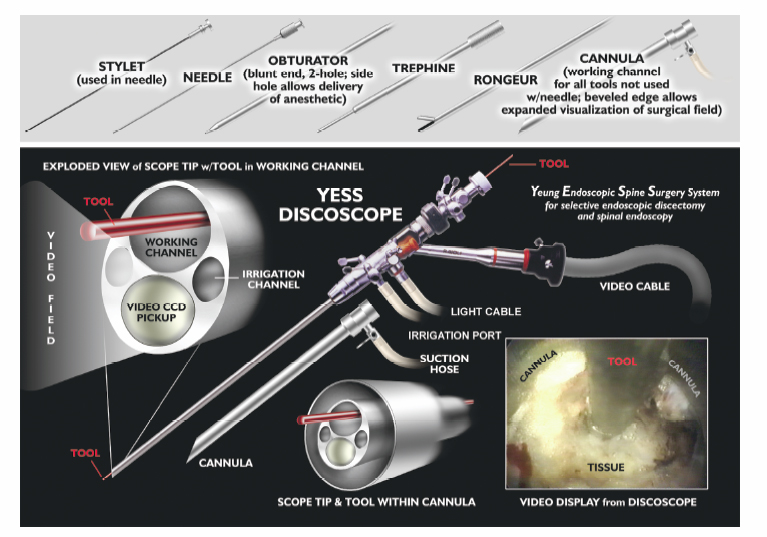
10 Percutaneous Decompression Christopher A. Yeung, Christopher P. Kauffman and Anthony T. Yeung Throughout much of the late 20th century, surgical specialists have pursued less invasive techniques for the treatment of spinal problems. The patient benefits from these less invasive techniques have been well documented through the literature and have become the gold standard for numerous surgical procedures. Advances in the ability to perform endoscopic discectomy have paralleled these other specialties; yet percutaneous spinal surgery has not met with the same peer recognition as the other fields. This is due, in part, to the high success rate and relative low morbidity of the current gold standard, posterior microscopic lumbar discectomy. This approach, however, still requires a 1-inch midline or paramedian incision, muscle and ligament stripping, muscle retraction, partial facet and lamina resection, and neural retraction. This can weaken the muscular lumbar stabilizers, create instability and facet arthrosis, cause traction neuropraxia, promote epidural scarring, and make revision surgery more difficult. Today’s endoscopic technology allows for visualized discectomy and decompression of the traversing and exiting nerve roots from a percutaneous posterolateral/transforaminal approach. This is safe and equally efficacious to microscopic discectomy in properly selected patients. The patients may also reap the benefits of the less-invasive approach. Anterior and posterior interbody fusions are possible through percutaneous approaches. Pilot studies have begun using percutaneous delivery of autograft and allograft contained in an annular fence (Ouroboros Medical, San Diego, CA) and into a mesh bag (Optimesh, Spineology, St. Paul, MN) supplemented by facet or pedicle screw posterior stabilization. The progress made on percutaneous discectomy and decortication of the endplate will lead the way for future progress in percutaneous fusion. The percutaneous posterolateral endoscopic approach is also ideally suited for nucleus replacement and nucleus augmentation procedures. Future advances in the use of biomaterials and gene therapy may open the door to anular reinforcement and tissue repair or regeneration. The basis for percutaneous lumbar disc procedures stemmed from the use of a Craig needle to perform a posterolateral biopsy for neoplastic conditions.1,2 Percutaneous lumbar surgery is performed through what has been named the triangular working zone, or Kambin’s triangle (Fig. 10.1). This triangular zone is defined as a safe zone in the posterolateral anulus between the exiting and traversing nerve roots. The exiting nerve root forms the anterior border of the triangular zone as it exits under the cephalad pedicle. The superior endplate of the caudal vertebral body forms the inferior border, and the articular process and superior articulating facet of the caudal vertebra form the posterior border. The working zone is bordered medially by the traversing nerve root and dura. From cadaveric measurements it was determined that cannulas ranging from 4 to 10 mm could be safely used in the triangular working zone.3–6 A thorough knowledge of the three-dimensional anatomy is necessary to understand and perform posterior percutaneous lumbar surgery. Lyman W. Smith, MD, pioneered the use of chymopapain for the enzymatic degradation of the nucleus pulposus (NP) and coined the term chemonucleolysis. After obtaining permission from the FDA in 1963, Smith reported his first 10 patients’ results in 1964. Two of the next 20 patients treated sustained paralysis.7–9 Following its introduction chymopapain has been used on an inconsistent basis. In 1982, the FDA approved the use of a new formulation of the drug, Chymodiactin (Knoll Pharmaceutical Company, North Olive, NJ). Satisfactory results were reported in 82% of treated patients; however, media attention focused on 55 patients out of 100,000 who had catastrophic complications, 6 of them resulting in paraplegia secondary to acute transverse myelitis. Nordby et al10 reviewed the adverse effects of chymopapain over a 10-year history. In this period, most of the complications occurred from 1982 to 1984, with a 50% decrease in complications from 1984 to 1988. The early experience with higher complications was attributed to poor technique, including injection of chymopapain directly into the subarachnoid space. After the development and use of preoperative sensitivity testing and preinjection discography, the complication rate has dropped significantly.10 Fig. 10.1 (A)Triangular working zone at L5-S1 (Kambin’s triangle; area of stippling). This is the access point for posterolateral disc access. (B) Instrument entry angle in Kambin’s triangle. Accessory branches of the nerves can be found here and are named Furcal nerves. Chymopapain is the only percutaneous modality to date to undergo prospective double-blind randomized trials.11 The longest follow-up comes from an Australian study comparing chymopapain with saline injection. At 10 years, the patients remained blinded to which treatment they had received. The results showed that 80% of the patients who received chymopapain considered their surgery successful compared with only 34% of patients who received saline injection. Twenty percent of the chymopapain-treated patients went on to have open surgical intervention versus 47% of the patients receiving saline.11 Other studies comparing chemonucleolysis with placebo injections reported success rates from 71 to 80%. 12–14 There have been indirect comparisons of open discectomy and chemonucleolysis in the literature, but few direct comparisons. One small study prospectively compared the two. There were 46 patients in each group. Early results favored surgery, although at 1 year the two groups showed no statistically significant difference. One significant difference was that nine patients receiving chymopapain went on to have surgical intervention and only one in the surgical group underwent reexploration.15 In a review of 45 clinical studies involving over 7000 patients, Nordby et al10 reported an average success rate of 76% for chemonucleolysis versus an average of 88% for laminectomy and discectomy. In a meta-analysis of 43,662 patients treated with chemonucleolysis the overall complication rate was 3.7% with only 0.45% being severe complications. The study also looked at 2051 surgical patients with an overall complication rate of 26% and a severe complication rate of 4.2%.16 Currently, chymopapain is not commercially available in the United States. Onik et al17 introduced automated percutaneous lumbar discectomy (APLD), in 1985, after developing an automated Nucleotome (Clarus Medical, LLC, Minneapolis, MN). The procedure involved introduction of an 8-inch Nucleotome with a blunt end into the center of the disc space. There were three available diameters: 2.0, 2.5, and 3.5 mm. The Nucleotome had a cutting blade and suction. It was driven by nitrogen gas and operated under fluoroscopic control. There was no direct visualization of the disc space or neural structures. It was operated in the disc space for —10 minutes.17,18 The results of APLD have been mixed in the literature with few authors other than Onik et al being able to achieve their reported results. Initial results were 80% good to excellent, but only over a 6-month follow-up.19 A multi-center study reported a 75% success rate when proper inclusion criteria were met.20 The difficulty comes in interpretation of the imaging studies as they relate to contained disc herniations. Many of the failures were determined to be in noncontained herniations. In retrospectively reviewing their results at 5-year follow-up, Maroon found a success rate of 59%.18 Chatterjee et al21 performed a randomized prospective trial comparing APLD and microdiscectomy for contained herniations. The herniations could be no larger than 30% of the canal size. The results of this study demonstrated a success rate of 29% for APLD versus 80% for microdiscectomy. The authors concluded that APLD was not as successful for small, contained herniations as previously reported.21 In a separate study out of the United Kingdom, Grevitt et al23 reported on the long-term follow-up of initially successful APLD patients. The original study group had a 72% good to excellent result.22 However, on longer follow-up (55 months), 33% of patients deteriorated into a fair or poor group making the overall success rate 45%.23 Another study of long-term results showed that 38% of patients required subsequent open surgery within 5 years.24 The use of a laser (light amplification by stimulated emission of radiation) is predicated on the ability of the laser to ablate tissue and achieve hemostasis. Lasers have been used extensively throughout multiple surgical specialties with success. There are several types of lasers used in medicine, including infrared, light, and ultraviolet lasers. Lasers that have been used successfully in the lumbar spine include the CO2, potassium titanyl phosphate (KTP), Holmium: yttrium-aluminum-garnet (YAG), and neodymium:YAG. Only the KTP laser and the Holmium:YAG laser are FDA approved for use in the spine. The underlying principle of laser lumbar surgery was that through tissue ablation intradiscal pressure could be substantially lowered. This was based on the work of Hirsh et al25 and their postulated relationship between intradiscal pressure, disc herniation, and low back pain. They hypothesized that lowering this pressure in an injured disc could be efficacious in the relief of sciatica.25 Based on this and early results of chymopapain and APLD, studies were performed to see how much disc could be ablated with the laser. Multiple studies described decreases in intradiscal pressures of 50% or greater.26–28 The results of laser discectomy fall within a similar range to that of chemonucleolysis and early results of APLD. Using the KTP laser, Davis28a reported a success rate of 80%. In a larger study, Choy and colleagues looked at 333 patients with herniated discs diagnosed by magnetic resonance imaging (MRI) or computed tomography (CT) and treated with percutaneous laser discectomy. At an average follow-up of 26 months, they reported a 78.4% overall success rate.29 Percutaneous laser discectomy, like its predecessors, was a fluoroscopically guided, nondirectly visualized procedure performed with a laser guide or partially visualized with smaller endoscopes. The inability to see the decompressed nerve or the targeted pathoanatomy consistently has limited its use. At this time, with newer operating or working channel spine endoscopes, the laser is used mainly as an adjunctive tool for hemostasis and targeted tissue ablation during endoscopic lumbar procedures. Endoscopic surgery developed out of fluoroscopically guided percutaneous procedures that initially used working cannula with modified instruments designed for disc removal. The first surgeon credited with percutaneous nucleotomy was Hijikata in 1975.30 The evolution of endoscopic techniques followed a series of transitions. Initially, an arthroscope was used to inspect the disc and anulus intermittently through the cannula, while the mechanical nuclectomy was done under fluoroscopic guidance. The introduction of a biportal approach allowed for direct visualization of instruments introduced through a cannula inserted into the disc from the opposite posterolateral portal. The later development of an operating spine scope with a working channel allowed for surgical removal of disc material and visualization of foraminal anatomy under direct visualization via a uniportal approach. Parviz Kambin performed the first true endoscopic lumbar procedures. The arthroscope was at first used intermittently through the working cannula. At certain stages of the procedure, such as perforating the disc in the triangular working zone, the arthroscope would be placed in the cannula. The nonworking channel scope was used for identification of the anulus and periannular structures and to ensure the nerve was not in the way prior to advancement of the cannula. Once the cannula was safely within the disc, the Nucleotome, an arthroscopic shaver, and pituitary rongeurs were passed through the cannula to perform mechanical disc removal. The majority of the procedure was only fluoroscopically visualized.31 Kambin reported an 88% success rate in his first 100 patients.32,33 The early endoscopic procedures were limited by the absence of a working channel arthroscope. This led Kambin to the development of a biportal technique in which the scope was inserted on one side and the working cannula on the opposite side. Kambin’s indications for a biportal approach included large subligamentous herniations, extraligamentous herniations, and arthroscopic interbody fusion.5 In later studies, Kambin reports results from both uniportal and biportal procedures together. Overall results ranged from 85 to 92% satisfactory results at a minimum 2-year follow-up. There was no differentiation made between the results of uniportal versus biportal approaches.34–36 Kambin’s first prototype of the working channel scope was not fully developed and was not successfully marketed. The problems with the initial scope included fragility, limited degree of angulation for the working instruments, and the inability to establish adequate inflow or outflow for adequate visualization.37 Anthony Yeung developed the first working channel endoscope to become widely available. The scope was developed in 1997 and was approved for use by the FDA in March of 1998. The YESS (Yeung endoscopic spine surgery) system (Richard Wolf Surgical Instruments, Vernon Hills, IL) modified the scope by adding multichannel integrated irrigation, specialized beveled cannulas, a two-hole obturator, and newly designed discectomy tools, which allowed for constant real-time visualization with a uniportal technique (Fig. 10.2).38 Another major change, which allowed for advancement in the field of endoscopic spinal surgery, was the emphasis on placement of the cannula closer to the epidural space and the base of the targeted disc herniation. This enabled surgeons to target extruded herniations in addition to contained herniations. Previous percutaneous modalities all focused on entry through Kambin’s triangle and working within the center of the disc with the cannula anchored inside the anulus. The cannula was advanced past the anulus and remained there under fluoroscopic control. Matthews’ transforaminal approach for microdiscectomy allowed for routine visualization of the epidural space and greater access to the traversing nerve root.39 The development of a working channel scope and use of the transforaminal approach utilizing beveled and slotted cannulas enhanced endoscopic lumbar surgery. Using this approach, surgeons are able to operate under full visualization throughout most of the procedure and follow the neural structures into the epidural space. The specialized cannulas provide greater access to pathology; and they help protect and retract sensitive anatomy such as the exiting nerve and dorsal root ganglion. The working channel also allowed the passage of high-speed burrs for bone removal and direct foraminal enlargement and decompression of foraminal stenosis (foraminoplasty) (Fig. 10.3). Current indications for the use of an endoscopic posterolateral approach to the lumbar spine include foraminal and far-lateral disc herniations, contained central and paracentral disc herniations, small nonsequestered extruded disc herniations, recurrent herniations, symptomatic annular tears, synovial cysts, biopsy and debridement of discitis, decompression of foraminal stenosis with or without spondylolisthesis, visualized total nuclectomy (prior to nucleus replacement), visualized discectomy, and endplate preparation prior to interbody fusion. Perhaps the ideal lesions for posterolateral selective endoscopic discectomy are the foraminal and extraforaminal disc herniations. The cannula inserts directly at the herniation site and the exiting nerve is routinely visualized and protected. This approach requires less manipulation of the exiting nerve root than the paramedian posterior approach. Any herniation contiguous with the disc space not sequestered and migrated is amenable to endoscopic disc excision if the bony anatomy permits an unobstructed approach. This utilizes an “inside-out” technique in which the herniation is grasped from its base within the disc space, pulled back into the working intradiscal cavity, and removed via the cannula. The size and types of herniations chosen by the surgeon for endoscopic excision will depend on the skill and experience of the surgeon. Certainly, all contained disc herniations are appropriate for endoscopic decompression. With experience, extruded herniations can be routinely addressed. This approach is especially attractive for recurrent herniations after a traditional posterior approach because the surgeon can avoid the scar tissue from the previous surgery.
History
Anatomy
Types of Percutaneous Lumbar Discectomy
Enzymatic Discectomy
Automated Percutaneous Lumbar Discectomy
Percutaneous Laser Discectomy
Endoscopic Lumbar Discectomy
Indications and Contraindications
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








