Fig. 1
Microsurgical instruments (①) and the filament (② and ③) used in inducing SAH
A set of micro-neurosurgical apparatus included a microscope, microsurgical scissors, elbow microsurgical forceps, straight microsurgical forceps, bipolar coagulation, and micro-clips. Other equipment included a razor for shaving animal fur, normal forceps, ophthalmological scissors, needle-holder, scalpel, and sutures. Filaments (ShaDong Biological Tech. Co., Ltd., Beijing) marked with a certain scale were smoothed to blunt the top in advance under the microscope. Small cotton balls and gauzes were prepared to stop bleeding during the operation.
Anesthesia
C57BL/6 mice were anesthetized with intraperitoneal administration of 0.2 mL/100 g of a mixed solution consisting of 5 mg/mL ketamine and 2.5 mg/mL xylazine. Under anesthesia, the mice stayed still and unresponsive to any external stimuli, including cutaneous pinching and tail pinching with a sharp forceps, which the mouse would withdraw when pinched. For the righting reflex, mice did not right themselves from dorsal to sternal recumbency and muscles were relaxed when picked up by the tail. Mice had constant breath and heart beat. The absence of the palpebral reflex is considered a respectable depth. Because body temperature is reported to suddenly decrease after induction and remains diminished during anesthesia, hypothermia is considered a potentially fatal injury in small rodents. Therefore, maintenance of body temperature was included in anesthesia management. Mice were placed on a heating pad that was preheated to 37 °C and maintained until termination of surgery [1, 8].
Operation Procedures (Fig. 2)
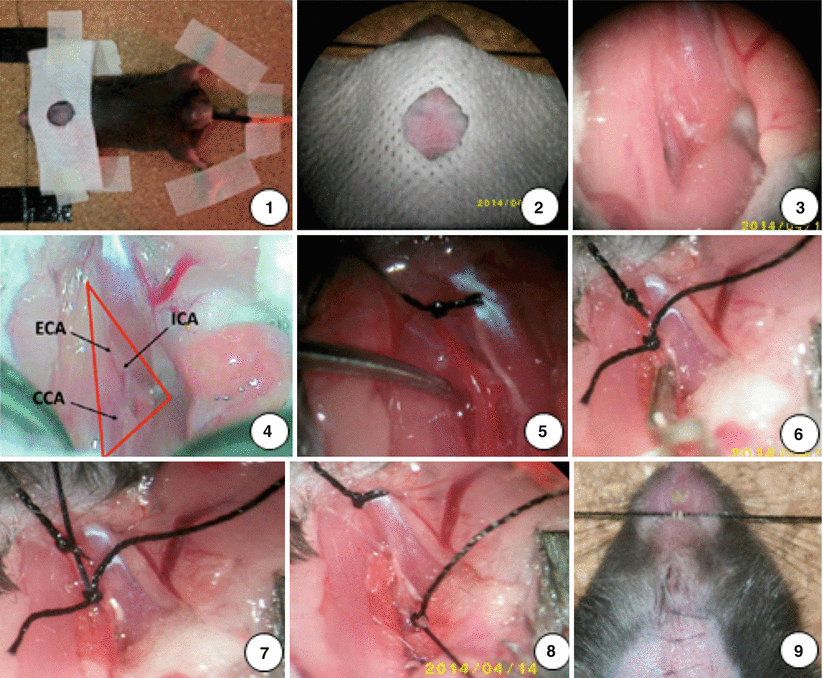
Fig. 2
Surgical steps for inducing SAH model in the mouse (as described in the text)
Mice were fixed in a supine position and shaved of neck fur. A rectal temperature probe was inserted to monitor and maintain the body constantly at 37 °C with a heating pad during surgery. The neck region was disinfected with 70 % ethanol. Under a microscope (Zeiss), a midline neck incision was made and the skin was cut with a pair of scissors from sternum to chin (1 cm). The surrounding tissue was dissected bluntly and the salivary glands pushed aside. The left common carotid artery (CCA) was exposed and gently mobilized. Great care was taken not to harm the vagal nerve, which runs in the same surrounding tissue sheath as the CCA. Using the same technique, the left external carotid artery (ECA) was exposed and mobilized. The first ECA branch, which is the occipital artery, was mobilized and coagulated. The ECA was ligated as far cranially as possible and pulled to the other side of long ligation to the right side and fixed with adhesive plaster. Two 5–0 silk sutures were prearranged (1.5 cm segments) for the filament around the ECA. The distal of the left internal carotid artery (ICA) was gently exposed. The origin of the ECA was temporarily occluded with one micro-clip. A hole was cut as near as possible to the previous ligation for the filament insertion with a vessel micro-scissors. A 20-mm-long blunted 5–0 monofilament nylon suture was inserted into the ECA. Two prearranged sutures were knotted with the appropriate intensity and then the ECA was cut at thr hole point. The micro-clip from the ECA was removed and the filament was advanced with a forceps into the ICA to its bifurcations, where resistance was encountered. The filament was immediately withdrawn after advancing another 2 mm further to perforate the vessel. The total length inserted into the artery was defined to be 10 mm. The ECA was ligated by knotting the prearranged ligations tightly. The skin wound was closed immediately with suture after the removal of the filament. Mice were continuously observed under 23 °C until recovery and were then returned to their cages. Subcutaneous injections of 0.5 ml saline were given twice per day to all mice to standardize hydration. All of SAH mice were analgesized with buprenorphine (0.03 mg/kg body weight intraperitoneally) once per day for at least three days after surgery [3, 14, 17].
Mouse Motor and Sensory Scale (MMSS)
The Mouse Motor and Sensory Scale (MMSS) was used to evaluate the neurologic deficits of SAH mice before operation and at day 3, 7, and 14 after SAH induction [15]. The scale was combined from the prior scales in previously described examinations [4, 7, 9], which were comprised of motor (0–12) (spontaneous activity, symmetry of limb movements, climbing, balance) and sensory (5–15) (proprioception, vibrissae, visual, olfactory, and tactile responses) (Table 1). Neurologic function is graded on a scale of 5–27, with 5 indicating maximum functional deficits and 27 normal neurologic functions; the lower the score, the more severe the brain injury.
Function | 0 | 1 | 2 | 3 | |
|---|---|---|---|---|---|
Motor | Activity (5 min open field) | No movement | Moves, no walls approached | 1–2 walls approached | 3–4 walls approached |
Limb symmetry (suspended by tail) | Left forelimb, no movement | Minimal movement | Abnormal forelimb walk | Symmetrical extension | |
Climbing (on inverted metal mesh) | Fails to hold | Hold < 4 s | Holds, no displacement | Displaces across mesh | |
Balance | Falls < 2 s | Falls > 2 s | Holds, no displacement | Displaces across rod | |
Sensory | Proprioception (cotton tip to both sides of neck) | No reaction | Asymmetrical head turning | Symmetric head turning | |
Vibrissae (cotton tip to vibrissae) | No reaction | Asymmetrical head turning | Symmetric head turning | ||
Visual (tip toward each eye) | No reaction | Unilateral blink | Bilateral blink | ||
Olfactory (lemon juice on tip) | No sniffing | Brief sniff | Sniff >2 s | ||
Tactile
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





