and Aditya G. Shivane1
(1)
Cellular and Anatomical Pathology Level 4, Derriford Hospital, Plymouth, UK
Abstract
Peripheral nerve diseases are very uncommon, except in the elderly population, and in many cases the aetiology remains unknown despite investigations. In certain situations a nerve biopsy may be helpful in the investigation of patients, particularly where there is a reasonable likelihood of detecting a treatable disorder, and a skin biopsy may also help in confirming the presence of a small fibre neuropathy. The techniques for nerve biopsy and laboratory investigation are described, along with a detailed classification of peripheral neuropathies and their key pathological findings.
Keywords
Peripheral neuropathyNerve biopsySkin biopsySmall fibre neuropathyCharcot Marie-Tooth diseaseDisorders of peripheral nerves affect approximately 0.5 % of the population, although this is higher in the elderly group. Neuropathies may be caused by a wide range of different aetiological factors ranging from genetic, metabolic, toxic, infective, inflammatory and neoplastic/paraneoplastic (Table 10.1), but in many cases the pathology is not specific. Investigation of peripheral nerve disease includes a thorough clinical history, examination, family history, nerve conduction studies and blood investigations, particularly to exclude potentially treatable metabolic, toxic and nutritional causes. Generally nerve biopsy has a low diagnostic yield in slowly progressive chronic symmetric axonal neuropathies, and highest in rapidly progressive and asymmetric neuropathies. A nerve biopsy is particularly helpful in confirming peripheral nerve vasculitis, paraproteinaemic neuropathy, amyloid neuropathy (although rectal biopsy and abdominal fat biopsy should be considered first), primary neuritic leprosy, granulomatous disease such as sarcoidosis, and neoplastic infiltration. Nerve biopsy may also be helpful in supporting the clinical diagnosis in some cases of atypical chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). In some storage disorders, genetic and toxic neuropathies, nerve biopsy may show characteristic changes, however, most of these conditions can be diagnosed by other means.
Table 10.1
Classification of peripheral neuropathies
Inherited |
Hereditary sensory and autonomic neuropathy (HSAN) |
Hereditary motor and sensory neuropathy (HMSN) |
Congenital demyelinating neuropathy |
Hereditary neuropathy with liability to pressure palsies (HNPP) |
Giant axonal neuropathy |
Refsum’s disease |
Hereditary amyloidosis |
Leukodystrophies |
Porphyria |
Metabolic |
Diabetes mellitus |
Vitamin deficiencies (thiamine, cobalamin, folate, vitamin E) |
Uraemia |
Hypothyroidism/hyperthyroidism |
Acromegaly |
Toxic |
Alcohol |
Lead |
Drugs |
Critical illness neuromyopathy |
Inflammatory |
Guillain-Barre syndrome |
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) |
Vasculitis |
Sarcoidosis |
Idiopathic perineuritis |
Infective |
Leprosy |
HIV infection |
Lyme disease |
Cytomegalovirus |
Diphtheria |
Neoplastic/paraneoplastic |
Primary/light chain-associated amyloidosis |
Paraproteinaemic neuropathy |
Skin biopsies have also been recently introduced as a relatively simple technique for confirming small fibre neuropathies, which has a low morbidity and can be repeated if necessary. It may also have value in confirming a diagnosis of idiopathic Parkinson’s disease [1], and if taken from glabrous skin can provide information about demyelination [2] and possibly vasculitis [3].
10.1 Nerve Biopsy Technique
Nerve biopsies can be undertaken under local anaesthesia from a variety of peripheral sites although the sural nerve and superficial peroneal nerve are the most commonly sampled. It is often helpful to take an additional sample of adjacent skeletal muscle as this does not significantly increase the morbidity and may increase the likelihood of diagnosing vasculitis, amyloid or sarcoidosis. Muscle can be taken through the same incision, to sample peroneus brevis when taking the superficial peroneal nerve, or gastrocnemius when sampling the sural nerve in the mid calf. Other nerves that are sometimes biopsied include the superficial radial nerve in pure upper limb neuropathies, and the obturator or musculocutaneous nerve in pure motor neuropathies. The nerves sampled should be clinically affected and great care must be taken during removal to avoid any pressure, stretching, kinking or cautery, as peripheral nerves are very sensitive to traumatic artefact which will limit their value for histological assessment. Samples should be rapidly transferred to the laboratory for fixation and freezing. Nerve biopsies carry a significant morbidity including permanent sensory loss in the distribution of the nerve sampled, and 30–40 % of patients will develop longstanding unpleasant sensations including dysaesthesias, paraesthesias and pain. Around 1 % of patients will develop a neuroma. As many of the neuropathies which can be detected in a nerve biopsy are patchy, 3–5 cm of nerve should be sampled to increase the likelihood of detection. A fascicular nerve biopsy will result in a smaller sample and does not appear to affect the complications [4].
10.2 Laboratory Preparation
Nerve biopsies should only be handled in specialist laboratories with suitably trained staff. It is crucial that they are fixed carefully and orientated correctly in order to maximise the information that can be obtained. Usually about half of the biopsy is fixed in formalin for paraffin histology, and subsequently stained with haematoxylin and eosin, congo red stain (for amyloid) and Perl’s preparation (for iron which is deposited around blood vessels in vasculitis). Paraffin histology is particularly useful for diagnosing vasculitis or amyloid deposition. A small disc from the tip of the biopsy is used for freezing which allows genetic testing and immunofluorescence, which can be useful for demonstrating paraprotein and complement deposition within peripheral nerves. The remainder of the tissue is fixed in glutaraldehyde, which is then used to make resin embedded sections, which are usually stained with toluidine blue, and processed for electron microscopy. This provides the most detailed histological preservation to allow assessment of fibre density and demonstration of axonal and demyelinating changes. Electron microscopy is particularly useful for examining unmyelinated fibres and can demonstrate characteristic abnormalities, including widely spaced myelin lamellae and inclusions in some cases of toxic neuropathy (e.g. amiodarone and chloroquine). In addition the glutaraldehyde fixed material is suitable for nerve fibre teasing (manually dissecting fascicles into individual myelinated fibres) which is the most sensitive way to demonstrate demyelination and can demonstrate structures such as tomaculae, in hereditary neuropathy with liability to pressure palsies.
10.3 Normal Structure of Peripheral Nerve
The basic structure of peripheral nerve is described in Chap. 1. Myelinated nerve fibres range in size from 2 to 17 μm in diameter, but have a bimodal distribution with small diameter myelinated fibres approximately 5 μm in diameter and larger fibres around 10 μm in diameter. The proportion of small and large diameter fibres varies between nerves, as does the density. In the sural nerve the myelinated fibre density ranges from around 5,000–8,000/mm2, with an age dependent decline. The thickness of the myelin sheath is proportionate to the axon diameter and the ratio of axon diameter to total nerve fibre diameter is known as the G-ratio (normally is between 0.5 and 0.7). The G-ratio may increase in demyelinating neuropathies, where there is remyelination, which results in a thinner myelin sheath than expected for the size of the axon. Each myelinated segment is associated with a Schwann cell, which produces and maintains the myelin. Within the nerve fascicle there are large numbers of unmyelinated fibres, which occur in clusters surrounded by non-myelinating Schwann cells (Remak cells), and are best seen at electron microscopy (see Chap. 1).
10.4 General Pathological Reactions
Peripheral nerves generally show two major patterns of pathology: axonal degeneration and segmental demyelination. The majority of peripheral neuropathies are characterised by an axonal pattern of degeneration, with Wallerian type distal degeneration with the formation of ovoids containing myelin and axonal debris eventually ingested by Schwann cells and macrophages. Wallerian degeneration extends proximally to the next intact node of Ranvier, from which the nerve may attempt regeneration by sprouting new axons to form a regenerating cluster (Fig. 10.1). Axonal neuropathies may also be characterised by distal dieback phenomenon, with axonal atrophy and degeneration with relatively little regeneration. Following Wallerian degeneration, the neuronal cell body may undergo chromatolysis several days later, with loss of its Nissl substance and peripheral displacement of the nucleus.
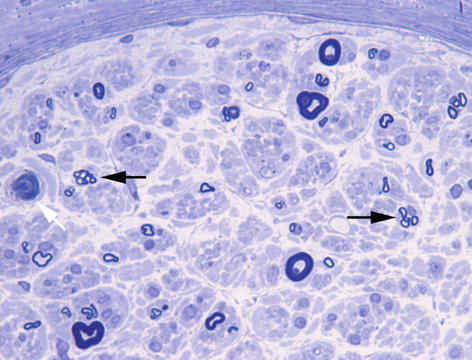

Fig. 10.1
Chronic axonal neuropathy showing scattered regenerating clusters (black arrows) and an occasional acutely degeneration fibre (white arrow). Toluidine blue stain, resin embedded section
Demyelinating neuropathies are characterised by primary loss of myelin sheaths, which may show regeneration, which results in inappropriately thin myelin sheaths and short internodes. Repeated episodes of demyelination and remyelination result in the formation concentric rings resembling onion bulbs (Fig. 10.2). It should be noted that demyelinated neuropathies may eventually result in axonal loss, and in some axonal neuropathies, an element of demyelination may be seen.
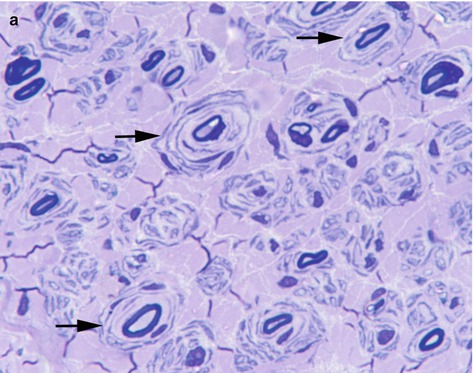
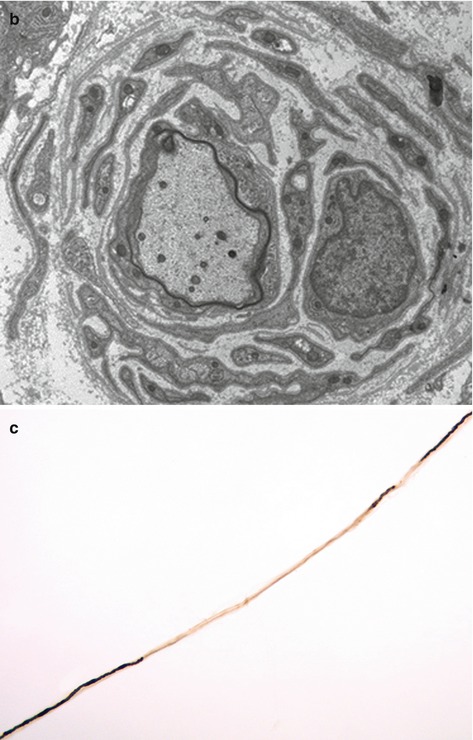


Fig. 10.2
(a) Chronic demyelinating neuropathy showing the formation of onion-bulbs (arrows). Toluidine blue stain, resin embedded section. (b) Onion bulb composed of concentrically arranged schwann cells surrounding a central axon, which is partly covered by thin myelin. Electron microscopy. (c) Fibre showing segmental demyelination. Teased fibre preparation
10.5 Inflammatory Neuropathies
10.5.1 Guillain-Barre Syndrome
This is an acute or subacute progressive polyneuropathy that may follow a non-specific upper respiratory or gastrointestinal infection (e.g. cytomegalovirus, campylobacter jejuni, Epstein-Barr virus, mycoplasma pneumonia and human immunodeficiency virus), vaccination, pregnancy or surgery. Typically it causes paraesthesia followed by weakness, but variants include pure motor or sensory syndromes, autonomic dysfunction and the Miller Fisher syndrome (ataxia, areflexia and opthalmoplegia). Neurophysiology usually shows evidence of slowed nerve conduction and CSF protein is elevated. In most cases a nerve biopsy is not indicated. The disease is characterised by patchy foci of segmental demyelination, endoneurial oedema and patchy inflammation. A pathognomonic, but uncommonly seen, feature is macrophage-induced stripping of myelin sheaths, which can be seen at electron microscopy. The predominantly axonal forms (acute motor axonal neuropathy, acute motor and sensory axonal neuropathy), have a worse prognosis, and are often associated with campylobacter jejuni infection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








