Fig. 3.1
Validation of the neuronal origin of serotonin (5-HT) sampled by a microdialysis probe (exposed tip length, 1 mM) in the central nucleus of the amygdala (CeA) of freely moving rats. (a) Local administration of high concentrations of potassium through the probe (60 mM for 60 min). (b) Administration of tetrodotoxin (1 μM for 105 min) through the probe. (c) Perfusion with calcium-free Ringer supplemented with EGTA (1 mM for 105 min). (d) Local application of the 5-HT1B agonist RU 24969 (300 nM for 30 min). Measurements in the CeA were performed in the presence of 10 μM fluvoxamine (flow rate = 1.5 μl/min; sample time =15 min). Horizontal bars indicate perfusion with the test substance. The bars are corrected for the lag time in the microdialysis system. *significantly different from Ringer, Bonferroni contrast test following ANOVA; p < 0.05. Key: ■ Ringer (n = 7); ● potassium (n = 7); □ TTX (n = 7); ○ calcium-free Ringer (n = 7); ∆ RU 24969 (n = 4) (From Bosker et al. 1997)
3.3 Methodology of Microdialysis
Basically a microdialysis probe consists of an inlet and an outlet tube connected by a membrane. Performance of the membrane in terms of recovery and responsiveness greatly depends on its physical (molecular weight cutoff) and chemical (hydrophobicity) properties. The most popular Y-shaped microdialysis probe is a concentric design with an outer diameter of approximately 300 μm (see Fig. 3.2). Probes are inserted into the brains of laboratory animals at coordinates derived from a dedicated atlas (for instance, Paxinos and Watson 1986) using a stereotaxic instrument. In rodents, surgery takes place under anesthesia, and the animals are allowed to recover from surgery for at least 24 h before the microdialysis experiments commence.
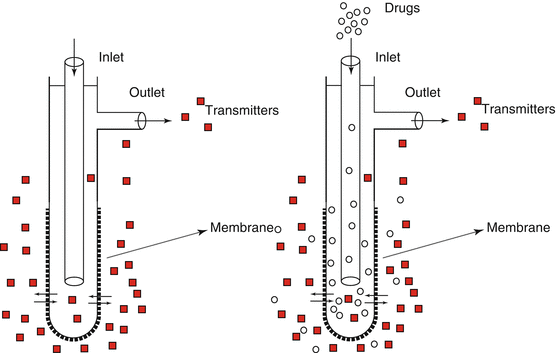

Fig. 3.2
Schematic representation of a Y-shaped microdialysis probe with both anterograde and retrograde perfusion
A microdialysis experiment begins by connecting the inlet of the probe via tubing to a high-performance perfusion pump carrying a syringe filled with Ringer solution (artificial CSF) to be perfused through the probe at a constant flow rate mostly in the range of 1–2 μl/min. It is important that membrane and tubing are essentially inert to minimize sticking of endogenous or exogenous compounds. It is common practice to perfuse the probe for 2 h prior to the actual microdialysis experiment to obtain a stable baseline. Samples can be collected in vials using a fraction collector for later analysis (off-line) or in an HPLC injection loop for immediate analysis (semi-online).
Theoretically, microdialysis does not involve exchange of fluid with brain tissue, but endogenous compounds (anterograde microdialysis) and exogenous compounds (retrograde microdialysis) are able to diffuse through the membrane driven by the concentration gradients between the extracellular fluid in the brain and the perfusion fluid pumped through the microdialysis probe (see Fig. 3.2). Except for glutamate, it is thus possible to directly measure the effects of most pharmacological interventions on the release of neurotransmitters. At the end of the experiments, the animals are sacrificed and the location of the probe is verified histologically. Monoamines can be analyzed with either HPLC and electrochemical detection or liquid chromatography with mass spectrometry (LC-MS). The latter method is more expensive but also more accurate and sensitive, and it allows the measurement of many neurotransmitters in one run, including glutamate and GABA.
It is important to note that the here-described methodology refers to rodents. For nonhuman primates (NHP), the methodology is different in several important areas. For instance, NHP are allowed to recover for at least 2 weeks before the microdialysis studies commence, and the artificial CSF for NHP is also different from that used in rodents and not always based on a Ringer solution. In addition, the pre-experimental perfusion is usually shorter (1 h), due to restrictions on how long the NHP can be restrained during the study. Finally, NHP are usually not sacrificed following these studies, and the location of the guide cannula is often verified via MRI. For more details, we refer to the microdialysis studies in rhesus monkeys by Bradberry (2002), Wilcox et al. (2005), Howell et al. (2006), Banks et al. (2009), (Andersen et al. 2010), Murnane et al. (2010); in squirrel monkeys by Czoty et al. (2002), Bauzo et al. (2009, 2012), Manvich et al. (2012); the review of the microdialysis methodology in NHP by Bradberry (2000); and the protocols by Saunders et al. (2001).
3.4 Pharmacological Interventions for Serotonin
It is common practice to manipulate serotonin levels in PET competition studies using serotonin reuptake inhibitors or releasers, but there are other options available as outlined below. Figure 3.3 schematically depicts various interactions of the serotonergic system with other neurotransmitters in the cell body area. However, such interactions may vary between brain areas and also the effect of pharmacological interventions on serotonin release, in particular between cell body and axon terminal areas.
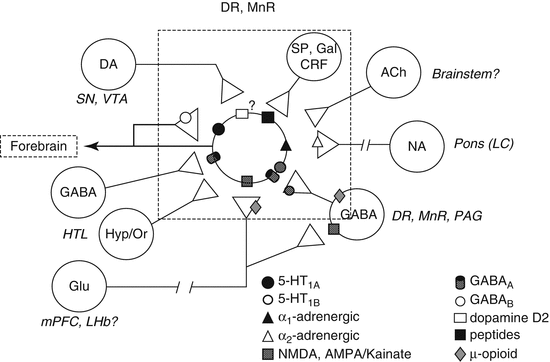

Fig. 3.3
Schematic depictions of serotonergic interactions with other neurotransmitter systems
3.4.1 Manipulation of Extracellular Serotonin Levels Through Synthesis and Reuptake Inhibition
A well-known strategy to manipulate serotonin levels in the brain is through the synthesis of the monoamine. Release and synthesis of serotonin depends on the availability of its precursor molecule, the essential amino acid tryptophan. This notion has been used to demonstrate the role of serotonin in antidepressant efficacy, as witnessed by the relapse of depressive symptoms following depletion of serotonin by tryptophan depletion in patients successfully treated with antidepressants (Delgado et al. 1990).
We have investigated the effects of 5-HT synthesis inhibition on the response to citalopram following retrograde infusion of the aromatic amino acid decarboxylase inhibitor NSD 1015 (3-[hydrazinomethyl] phenol dihydrochloride) and by oral tryptophan depletion. The microdialysis experiments in rats clearly show that inhibition of serotonin synthesis by local NSD 1015 infusion as well as tryptophan depletion significantly decreases the effect of an SSRI on extracellular serotonin levels (see Fig. 3.4a, b).
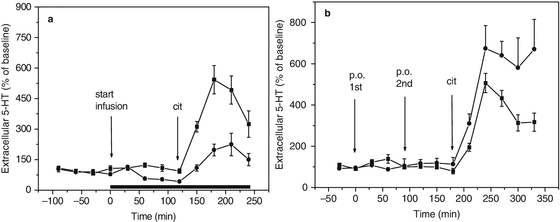

Fig. 3.4
(a) Microdialysis of 5-HT in hippocampus of freely moving rats (flow rate = 1.5 μl/min; sample time = 15 min). Effect of local 5-HT synthesis inhibition on the response to citalopram following retrograde infusion of the aromatic amino acid decarboxylase inhibitor NSD 1015 (3-[hydrazinomethyl] phenol dihydrochloride). ■: no synthesis inhibition, t = 120 citalopram 10 μmol/kg s.c.; ●: synthesis inhibition following local infusion of 10 μM of NSD 1015 at t = 0, t = 120 citalopram 10 μmol/kg s.c. (From Bosker et al. 2010). (b) Microdialysis of 5-HT in hippocampus of freely moving rats (flow rate = 1.5 μl/min; sample time = 15 min). Effect of oral tryptophan depletion on the response to citalopram. ■ low tryptophan, ● normal tryptophan. First arrow at t = 0: first oral administration of low tryptophan amino acid mixture; second arrow at t = 90: second oral administration; third arrow at t = 180: subcutaneous administration of citalopram 10 μmol/kg s.c. (From Bosker et al. 2010)
However, the effect of tryptophan depletion on basal serotonin levels was not significant. This indicates that synthesis might not be a limiting factor under normal conditions, but only when serotonin reuptake is inhibited (see also Bosker et al. 2010). The latter would be in agreement with a competition PET study using 18 F-MPPF, which could not demonstrate increased 5-HT1A receptor binding following tryptophan depletion in healthy volunteers (Udo de Haes et al. 2002).
Inversely, serotonin levels are only moderately increased following intraperitoneal administration of tryptophan in rats (Fig. 3.5a). However, when given prior to an SSRI, a strong dose-dependent augmentation of its effect on serotonin levels was observed (Fig. 3.5a). This also indicates that under normal conditions, synthesis is capable of maintaining serotonin levels but that reuptake inhibition puts an extra demand on synthesis.
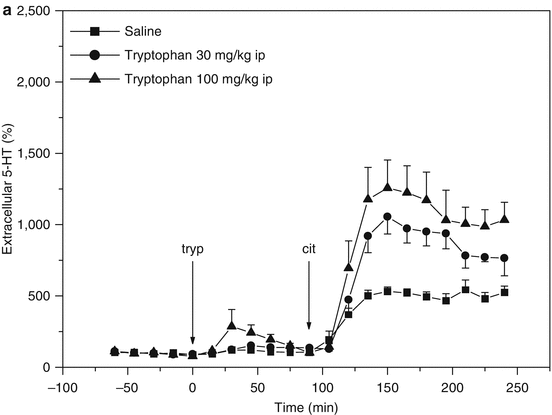
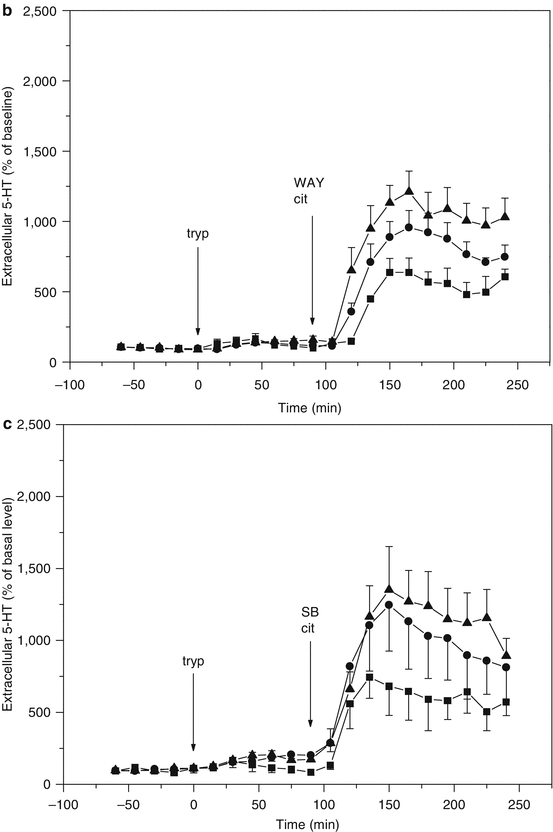
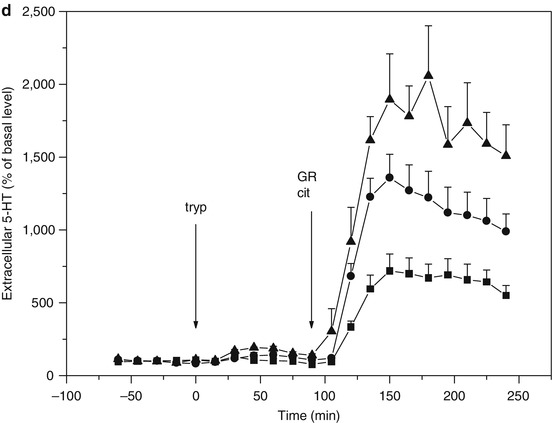



Fig. 3.5
(a) Microdialysis of serotonin (5-HT) in hippocampus of freely moving rats (flow rate = 1.5 μl/min; sample time = 15 min). Effect of tryptophan administration on the response to citalopram. ■ t = 0 saline i.p., t = 90 citalopram 10 μmol/kg s.c., ● t = 0 tryptophan 30 mg/kg i.p., t = 90 citalopram 10 μmol/kg s.c., ▲ t = 0 tryptophan 100 mg/kg i.p., t = 90 citalopram 10 μmol/kg s.c. (From Bosker et al. 2010). (b) Microdialysis of serotonin (5-HT) in hippocampus of freely moving rats (flow rate = 1.5 μl/min; sample time = 15 min). Citalopram and tryptophan administration synergistically increase 5-HT levels. Theoretically the effect is counteracted by an increased activation of somatodendritic 5-HT1A autoreceptors involved in feedback control of 5-HT synthesis and release (Cremers et al. 2000a, b and references therein), yet the effect of 5-HT1A antagonist WAY 100635 is only marginal. t = 90 citalopram (10 μmol/kg s.c.) and WAY 100.635 (1 μmol/kg s.c.). ■ t = 0 saline i.p., ● t = 0 tryptophan 30 mg/kg i.p., ▲ t = 0 tryptophan 100 mg/kg i.p. (From Bosker et al. 2010). (c) Microdialysis of serotonin (5-HT) in hippocampus of freely moving rats (flow rate = 1.5 μl/min; sample time = 15 min). Citalopram and tryptophan administration synergistically increase 5-HT levels. Theoretically the effect is counteracted by an increased activation of 5-HT2C receptors on GABA-B-ergic neurons involved in feedback control of 5-HT release (Cremers et al. 2007), yet the effect of 5-HT2C antagonist SB 242084 appears only marginal. t = 90 citalopram 10 μmol/kg s.c. and SB 242084 1 μmol/kg s.c. ■ t = 0 saline i.p., ● t = 0 tryptophan 30 mg/kg i.p., ▲ t = 0 tryptophan 100 mg/kg i.p. (From Bosker et al. 2010). (d) Microdialysis of serotonin (5-HT) in hippocampus of freely moving rats (flow rate = 1.5 μl/min; sample time = 15 min). Citalopram and tryptophan administration synergistically increase 5-HT levels. Theoretically the effect is counteracted by an increased activation of presynaptic 5-HT1B autoreceptors involved in feedback control of 5-HT synthesis and release (Cremers et al. 2000a, b and references therein), which is indeed supported by the marked effect of 5-HT1B antagonist GR 129735. t = 90 citalopram 10 μmol/kg s.c. and GR 129735 1 μmol/kg s.c. ■ t = 0 saline i.p., ● t = 0 tryptophan 30 mg/kg i.p., ▲ t = 0 tryptophan 100 mg/kg i.p. (From Bosker et al. 2010)
3.4.2 Manipulation of Extracellular Serotonin Levels Through Synthesis, Reuptake Inhibition, and Receptors Involved in the Regulation of Release and/or Synthesis
Synthesis and release of serotonin are controlled by somatodendritic 5-HT1A and presynaptic 5-HT1B autoreceptors. Blocking the 5-HT1A and 5-HT1B autoreceptors by, respectively, WAY 100.635 and GR 129735 augments the effect of the selective serotonin reuptake inhibitor citalopram on extracellular serotonin levels (Cremers et al. 2000a). Another form of negative feedback control of serotonin release is mediated by 5-HT2C receptors on GABA-B-ergic neurons (Cremers et al. 2007). Blocking the 5-HT2C receptors by SB 242084 augments the effect of the selective serotonin reuptake inhibitor citalopram on extracellular serotonin levels (Cremers et al. 2004). We have combined these SSRI augmentation strategies with tryptophan supplementation and monitored the effects on extracellular serotonin using microdialysis in hippocampus of freely moving rats (Fig. 3.5b, c, d).
SSRI augmentation with tryptophan in combination with inhibition of receptor-mediated feedback mechanisms seems a promising pharmacological intervention to increase extracellular serotonin levels in humans. The critical role of serotonin synthesis following reuptake inhibition and tryptophan supplementation is emphasized when serotonin levels are further increased using an augmentation strategy based on antagonism of 5-HT1B receptors involved in feedback control of serotonin synthesis and release (Fig. 3.5d).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





