Chapter 18 Physical activity and exercise in neurological rehabilitation
Terminology and definitions
 Performance-related fitness: Components of fitness necessary for optimal performance at work or sport (Bouchard et al., 2007).
Performance-related fitness: Components of fitness necessary for optimal performance at work or sport (Bouchard et al., 2007). Health-related fitness: Components of fitness that benefit from a physically active lifestyle and relate to health (Bouchard et al., 2007). These include cardiovascular endurance, muscular strength and endurance, flexibility and body composition (American College of Sports Medicine (ACSM), 2006).
Health-related fitness: Components of fitness that benefit from a physically active lifestyle and relate to health (Bouchard et al., 2007). These include cardiovascular endurance, muscular strength and endurance, flexibility and body composition (American College of Sports Medicine (ACSM), 2006).Introduction and context
Engaging in exercise and being physically active has shown substantial health benefits in the general population. There is now overwhelming evidence that active lifestyles reduce rates of hypertension, obesity, stroke, coronary artery disease, some cancers, diabetes and osteoporosis (Blair, 2007; Cluve, 2008). A change in lifestyle from inactivityto activity and participation in exercise is associated with a reduction in disease and reduced premature mortality. This suggests that for most of us it is never too late to start being active and change exercise behaviour because the benefits are achievable at any age (ACSM, 2006).
Individuals with neurological conditions are often challenged by movement difficulties and therefore adopt a more sedentary lifestyle with increasing muscle weakness and cardiovascular deconditioning. This exposes them to all the risk factors associated with inactivity, in addition to the risk factors that contributed to their conditions in the first place. A basic level of fitness is essential for carrying out activities of daily living. For example, light housework and carrying shopping requires approximately 40–50% of peak VO2. Housework of lower workload intensity requires a minimum of 32% of peak VO2 (Arnett et al., 2008). This chapter proposes that, just like the general population, individuals with neurological conditions also benefit from staying active and taking regular exercise. This chapter therefore provides an overview of activity and exercise in these patient groups and outlines the current evidence to support this aspect of patient management. Wherever possible, guidance for exercise prescription will be presented. Aerobic training, resisted muscle strengthening, constraint-induced movement therapy and treadmill training will be discussed in more detail. Also see ‘Exercise and Movement’ in Chapter 12.
Being active with a neurological condition
Most people know that being active has health benefits and yet the majority of the general population is not adequately active to benefit (Marcus et al., 2000). Levels of exercise adoption suggest that this is also true for people with neurological conditions. For example, peak oxygen consumption following stroke has been found to be as low as low as 50% of the capacity of age- and sex-matched sedentary controls (Pang et al., 2006). In many cases it may be the fact that a person has an impairment or disability which contributes to their inactivity. Serious life events, such as being diagnosed with a long-term condition, make it more difficult to adopt or maintain an adequate level of exercise (Oman & King, 2000). It is probably unrealistic to expect our patients to change typical behaviours of exercise after a stroke, acquired brain injury, or being diagnosed with Parkinson’s disease. However, it appears that it is exactly this type of behaviour change that is required in most of our patients if they are to experience what we know are the benefits of exercise. Therefore, we are seriously challenged to identify the barriers to exercise adoption (also see ‘Engagement with falls prevention’ in Ch. 20). We will also need to be able to design more appropriate exercise service models and prescribe specific exercises to meet the goals of the patient, or adapt more general exercise guidance to fit specific conditions.
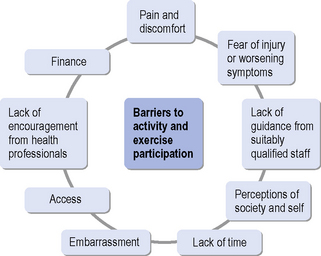
Kang (2007) found that young people with disabilities face many barriers to participating in or maintaining exercise behaviour. Barriers most frequently cited were a lack of time and actual pain and discomfort during exercise. Others were lack of facilities to exercise with peers, adverse weather and misconceptions by non-disabled people about the ability of the patient to take part in exercise. A better understanding of exercise ‘adherence models’ therefore may help service provision in the long term. It has recently been suggested that it is predominantly the intentions from the outset, described as the ‘Theory of Planned Behaviour’ (Yardley & Donovan-Hall, 2007), that determine exercise adherence and, to a lesser extent, symptom experience during exercise and rehabilitation. Very specific support in the form of counselling has also improved exercise adherence (van der Ploeg et al., 2006). A recent study (Elsworth et al., 2009) reported that people with neurological conditions would enjoy being active and taking part in exercise, but that they are mostly put off by embarrassment and the lack of well-educated staff with detailed knowledge of individual conditions. Group participation with people with similar disabilities was preferred and supervision by professionals with appropriate expertise was seen as a priority. This chapter therefore outlines the barriers that can relate to the individual and their personal concerns, the professional and support staff and their expertise, the facilities and transport and the overall service provision (see Figure 18.1).
Overcoming barriers to leading an active life and participating in exercise is a key challenge for rehabilitation therapists and knowledge of reported barriers is the first step in rising to this challenge. The reader is at this stage also reminded that physical activity and participation inexercise fit into the ICF model and framework described by the World Health Organization (WHO, 2001). According to this classification model, barriers to being active and taking part in exercise may well be related to the impairments caused by a particular pathological process. In addition, attitudes of society, inappropriate environments and a lack of adequate transport may make it difficult for some people to take part in regular exercise.
Opening opportunities for participation in exercise is therefore a specific challenge and some recent developments utilizing internet technology may offer alternatives to the more traditional fitness centre or rehabilitation facility. A range of small-scale studies has investigated the feasibility and potential outcome of home-based community exercise supported by internet technology. Often referred to as ‘telehealth’ or ‘telemedicine’, this approach seems appropriate in certain circumstances and may be of particular interest to individuals who live in more rural communities.Van den Berg et al. (2006) investigated the internet-based delivery of a home exercise programme for people with rheumatoid arthritis. Whilst the findings may not directly translate to people with neurological conditions, it is worth considering the lessons learned from this trial. The study compared two internet-based exercise programmes. One programme was individualized to the participant with tailored exercises and e-mail correspondence. The other only contained general exercise advice. The group receiving individualized exercise guidance was significantly better in achieving recommended exercise amounts.Finkelstein et al. (2008) evaluated the acceptability and effectiveness of a home-based telemedicine exercise programme in a small group of people with multiple sclerosis (MS). Support and guidance by a telehealth team over the 12-week exercise period showed significant improvements in walking ability and balance scores. Patients were also very satisfied with the service.
Hartley (2009) investigated the use of an exercise therapy model, largely based on patient self-management. The study showed that a self-management exercise programme for people with early MS was effective in improving gait and quality of life.
Measuring physical activity and fitness in neurological rehabilitation
Measurement and assessment of activity and physical fitness also fits into the ICF classification (WHO, 2001). Therapists are encouraged to choose appropriate assessment tools to capture the ability or difficulty of an individual to take part in physical activities. This may involve selecting either a global activity scale or, where available, a disease-specific measurement. Table 18.1 provides a selection of measurement scales to choose from. Guidance on a variety of rehabilitation measurements has been produced by Ainsworth (2009), Finch et al. (2002), Stokes (2009) and Bowling (2001).
Table 18.1 Activity measurement scales
| Activity scale/measurement | Reference | Comment |
|---|---|---|
| Barthel Index | Mahoney and Barthel, 1965 | Has been used in stroke, spinal cord injuries, MS and older people; good reliability in stroke |
| Functional Independence Measure (FIM) | Keith et al., 1987 | Has been used in MS, older people, stroke, head injury, Parkinson’s disease; reliability variable between conditions |
| Rivermead Mobility Index | Collen et al., 1991 | Developed for stroke patients and has shown reliability in this group |
| Baecke Activity Questionnaire | Voorips et al., 1991 | Designed for use in older people; has been used in Parkinson’s disease |
| Physical Activity Questionnaire (IPAQ) | Craig et al., 2003 | The questionnaire can be administered in different forms (e.g. telephone or interview) and has shown reliability |
More recently, a number of authors have evaluated the use of motion sensors in various patient groups, including some with neurological conditions. No comprehensive review on the use of these devices for people with neurological conditions currently exists and therefore there is yet no clear sense of the value of these instruments in neuro-rehabilitation. However, early signs suggest that these relatively simple and inexpensive devices may provide an objective measure of activity and therefore add to the often subjective nature of activity scales or patient recall. The group of motion sensors suggested for this purpose range from simple step counters to a variety of accelerometers. The technology has been evaluated in a group of older people by de Bruin et al. (2008)and their findings suggest that the technology is available and improving in terms of device reliability. Acceptability for the wearer varies between devices and must be considered carefully. Accelerometers have also been used in activity assessment in young people with cerebral palsy (Van der Slot et al., 2007), stroke patients (Hale et al., 2008) and in Parkinson’s disease (Skidmore et al., 2008). Common to all these studies was the relative ease of use and good reliability of measurements.
Measuring physical or health-related fitness requires a set of scales or tools, which will be different from activity measurements. Therapists or researchers are advised to consider carefully the nature of the impairment they may wish to evaluate and also the cost of measurement and the necessary skill required in conducting fitness measurements. A range of measurements and tools are available and Table 18.2 provides a selection of relevant fitness assessment tools. In many instances simple timed walking tests will provide a useful measure of walking-related fitness/ability which can be used to assess, review or screen patients. More complex measurements, particularly those that involve the measurement of metabolic systems, will of course provide an extensive range of information. However, conducting measurements of this nature requires specialist equipment and training, and may not add significantly to the patient assessment information. Therefore, therapists are advised to consider carefully what type of assessment information they require.
Table 18.2 Measurements and tests for physical and health-related fitness
| Exercise/ fitness measurement | Reference | Comment |
|---|---|---|
| Walking tests, e.g. | Finch et al., 2002 Mudge and Stott, 2007 | The various tests have been used with different patient groups. The reader is referred to Finch et al. (2002) or Mudge and Stott (2007) for a more detailed review |
| Physiological Cost Index | Danielsson et al., 2007 | Measures energy expenditure during walking; reliable in normal subjects but not fully consistent in stroke |
| Rating of perceived exertion | Borg, 1998 | Reliable and correlates well with heart rate and lactate thresholds |
| Graded exercise testing with metabolic test systems and protocols on treadmill or cycle ergometer | American College of Sports Medicine, 2006 | Objective measure of exercise capacity and helps identify limiting factors Measurements can be reliable and have been used in various patient groups |
Effects of exercise and physical activity
Evidence suggests that staying active and taking regular exercise has clear health benefits (Blair, 2007). There is now also a growing body of evidence showing that people with neurological conditions can benefit from exercise. There are now growing recommendations to include aspects of exercise training as part of the overall rehabilitation for people following spinal cord injury (Janssen & Hopman, 2005), stroke (Royal College of Physicians, 2004), MS (Rietberg et al., 2004), cerebral palsy (Rogers et al., 2008) and Parkinson’s disease (Skidmore et al., 2008). The effects of exercise are usually described in terms of acute and chronic effects on the cardiorespiratory and neuromuscular system. Table 18.3 briefly summarizes some of these general effects of exercise. For further details, the reader is referred to exercise physiology textbooks, such as McArdle et al. (2006). It is important to note that the response to exercise in people with neurological conditions will vary and therapists are advised to take this into account when prescribing exercise.
Table 18.3 Key effects and responses to exercise
| Exercise response and effect | Comment |
|---|---|
| Acute cardiorespiratory response | |
| Cardiorespiratory training effect | |
| Acute response to resisted muscle training | |
| Training effect of resisted muscle strengthening |
HR, heart rate; VO2, maximal oxygen uptake
The effects on the nervous system of exercise and its response have not undergone extensive research. However, it appears that this area would be of particular interest to patients with neurological conditions. A small body of knowledge exists which suggests that there may be effects of exercise which go beyond conditioning of the cardiorespiratory and muscular system. These effects have so far been studied in animal models under a limited number of conditions. These studies suggest that taking part in regular aerobic exercise may have a neuroprotective effect.Smith & Zigmond (2003) investigated this possibility in an animal model of Parkinson’s disease. Their study showed that forced physical activity in rats had a neuroprotective effect, believed to be due to a reduction in oxidative stress. Exercise may be able to slow the cognitive decline seen in Parkinson’s disease and also in Alzheimer’s disease (Dishman et al., 2006). Exercise enhances the calcium transport to the brain which in turn enhances dopamine synthesis. This may explain the potential for neuroprotection or restoration in Parkinson’s disease and dementia (Sutoo & Akiyama, 2003). An intensive 12-week ‘running’ activity in rats after middle cerebral artery occlusion has resulted in reduced infarct volumes, mediated via increased nerve growth factor (Ang et al., 2003). The authors concluded that physical exercise may cause neuroprotection following stroke. These studies are restricted to animal models but they point towards an exciting potential role of exercise in rehabilitation. This role may go beyond increasing fitness and strength for the purpose of improving function and quality of life. Exercise may protect the brain from some chronic diseases, such as Parkinson’s disease. Exercise may also have potential to facilitate brain plasticity following stroke. Basic research and clinical studies suggest that high intensity (i.e. high repetition, velocity and complexity) is a characteristic of exercise that may be important in promoting activity-dependent neuroplasticity of the injured brain (Nudo & Milliken, 1996).
Aerobic exercise training in neurological rehabilitation
Aerobic exercise programmes
Walking, either overground or treadmill, is a task-orientated aerobic exercise programme that emerges from the literature as a firm favourite. Overground programmes have the additional advantage of being easily accessible and of low cost. Ada et al. (2003b) showed that a community-based programme incorporating treadmill training alongside an overground walking programme significantly improved walking speed and walking capacity, although effects on disability were less clear. Trials involving treadmills have also demonstrated improvements ranging from improved gait speed and capacity (Macko et al., 2005) to changes in corticomotor excitablity (Fisher et al., 2008). Further detail and guidance for the use of treadmill training can be seen later in this chapter.
Another common aerobic approach utilizes cycle ergometry with functional and health-related quality of life improvements being demonstrated in a range of conditions including MS (Kileff & Ashburn, 2005; Mostert & Kesselring, 2002); spinal cord injury (Ditor et al., 2003; Hicks et al., 2003); and stroke (Tang et al., 2009). Aquatic exercise programmes are also popular and research has shown improvements in peak workload and function (Chu et al., 2004; Pariser et al., 2006).
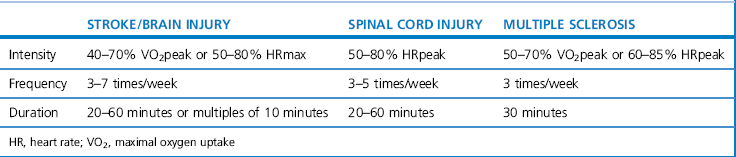
A combination of aerobic exercise, including walking, cycling and swimming, can result in positive treatment outcomes (Pang et al., 2006; Snook & Motl, 2009). Comprehensive guidance for prescribing aerobic exercise in neurological rehabilitation is currently incomplete and not fully based on high-quality evidence. Table 18.4 outlines some guidance for a number of conditions based on Gordon et al. (2004) and the ACSM (2003). The guidance is very broad and must be adapted to the individual after careful assessment.
Sports/exercise classes
People with spinal cord injury participating in sports have reported effects on aspects of health-related quality of life as well as facilitation of community reintegration by, for example, improved social reintegration networks or peer support (Labronici et al., 2000; Tasiemski et al., 2005). It is worth noting that Tasiemski et al. (2000) found that levels of participation in sporting activities decreased significantly after injury in people with spinal cord injury and that barriers to participation in these activities remain a complex issue, as illustrated in Figure 18.1 previously.
Therapists should also consider the use of other exercise classes such as tai chi, yoga or ballroom dancing. In older adults with chronic conditions tai chi appears to be safe and effective in promoting balance control, flexibility and cardiovascular fitness, alongside the psychosocial benefits (Wang et al., 2004). Tai chi has been trialled with people with Parkinson’s disease, but currently there is a lack of robust literature in this area (Lee et al., 2008). More recently yoga has been proposed as one of a range of mind–body therapies that could be of benefit to people with neurological conditions (Wahbeh et al., 2008). Researchers are also turning to innovative options such as dance, e.g. tango or American ballroom, which is showing promise in improving measures of walking, balance and quality of life measures in people with Parkinson’s disease (Hackney & Earhart, 2009).
Virtual reality training
Advances in virtual reality technology mean that devices using computer and gaming technology, such as the Nintendo Wii ®, are now found in many people’s homes. The potential of these types of adjuncts to maximize task-orientated practice and increase energy expenditure are beginning to be explored. The use of games using the Nintendo Wii ®, for example, has shown to increase energy expenditure in a group of asymptomatic participants (Graves et al., 2007; Lanningham-Forster et al., 2009). Research in individuals with neurological disorders, such as cerebral palsy, is beginning to emerge (Deutsch et al., 2008).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree