Fig. 13.1
Association between sedentary time (watching television and riding in a car) and development of depression. Adapted from Sui et al. (2015). The top panel shows the overall odds ratios and 95 % confidence intervals for risk of developing depression at 9.3 years of follow-up conferred by hours riding in a car, watching TV, and combined time riding in a car and watching TV at the baseline assessment. The lower section shows the risk (odds ratios and 95 % confidence intervals) for respondents who did and did not meet CDC physical activity guidelines at the baseline assessment. Low, moderate, and high levels of sedentary behavior were identified based on tertiles within the distribution. For riding in a car, low <5 h/week, moderate 5–8.9 h/week, high ≥9 h/week. For watching TV, low <5 h/week, moderate 5–10 h/week, high >10 h/week. For combined, low <12 h/week, moderate 12–18.9 h/week, high ≥19 h/week
The evidence for sedentary time for computer and internet use is somewhat mixed, suggesting that such time may not be a strong risk factor for depression. There may also be a circular pattern between sedentary time and depression. Not only does sedentary time appear to increase the risk of depression, but depression may lead to further increases in sedentary time (Teychenne, Abbott, Ball, & Salmon, 2014).
Physical activity and exercise interventions show positive effects on depression, and a number of reviews have been published summarizing study results. The American Psychiatric Association guidelines for treatment of patients with major depressive disorder cite physical activity as beneficial, particularly for individuals with co-occurring medical problems (American Psychiatric Association Work Group on Major Depressive Disorder, 2010). A review by Conn reported that physical activity interventions reduced depressive symptoms, even among adults who were not clinically depressed (Conn, 2010). Interventions ranged in duration, frequency, and session length, but typically were designed as 30–60 min sessions, 3 times a week, for a mean of 62 sessions. Standardized mean effect sizes were 0.372 and 0.522 for supervised and unsupervised physical activity interventions, respectively. Meta-analyses of interventions for adults who were clinically depressed have shown larger effect sizes, ranging from 0.72 to 1.42 (Conn, 2010). In a Cochrane review, exercise was associated with a greater reduction in depression than controls (standardized mean difference [SMD] −0.62, 95 % CI −0.81, −0.42) (Cooney et al., 2013; Cooney, Dwan, & Mead, 2014). A review by Teychenne concluded that both moderate- and vigorous-intensity interventions were effective, and that both center-based and home-based interventions were effective (Teychenne, Ball, & Salmon, 2008).
In the most recent review of randomized controlled trials of exercise interventions for depression , Stanton and Reaburn identified seven trials that met criteria for evaluation (included adults with depression diagnosed by DSM IV criteria or a validated depression scale; had an aerobic or resistance training program intervention of any duration; included a comparison group including pharmacotherapy, education, psychotherapy, other type of exercise [e.g., stretching], or no intervention; and used a validated depression scale as the outcome measure) (Stanton & Reaburn, 2014). The authors concluded that aerobic exercise programs at low-to-moderate intensity, performed 3–4 times per week, with sessions from 30 to 40 min, and a program duration of at least 9 weeks were beneficial in treating depression. They also noted that program adherence rates varied from 50 to 100 %, which may be due to the exercise format (group vs. individual), type of exercise, exercise intensity, or some combination of these factors. One of the reviewed studies did find that their higher intensity exercise had lower adherence (Trivedi et al., 2011); however, that same study found that the higher intensity program had higher depression remission rates. An earlier study found effects on depression only for a higher intensity exercise intervention that was equivalent to the CDC public health exercise guidelines (Dunn, Trivedi, Kampert, Clark, & Chambliss, 2005).
Reviewing exercise interventions among individuals with a variety of chronic conditions, including chronic pain and fibromyalgia, Herring also found that exercise reduced depressive symptoms (Herring, Puetz, O’Connor, & Dishman, 2012). Larger effects were noted when baseline depressive symptoms were higher and physical activity levels were moderate or vigorous (compared to low).
The effects of physical activity interventions on depression may be equivalent to the effects of psychological therapy and pharmacological treatment. In the Cochrane review previously cited, seven trials compared exercise with psychological therapy, and no difference was seen between the two interventions (SMD −0.03, 95 % CI −0.32, 0.26). Four trials compared exercise with pharmacological treatment, and again, no significant difference was seen between the two types of interventions (SMD −0.11, 95 % CI −0.34, 0.12) (Cooney et al., 2013, 2014).
Rheumatic Conditions
The findings in general population studies are replicated in studies of persons with arthritis. Kelley and colleagues conducted a systematic review of exercise intervention studies in rheumatic conditions that targeted depression (Kelley, Kelley, & Hootman, 2015). To be included in the review, studies had to report randomized controlled trials, include an exercise-only intervention of at least 4 weeks and a comparable control group, and include adults with osteoarthritis (OA), rheumatoid arthritis (RA), fibromyalgia, or lupus. Interventions needed to be “community-deliverable,” defined as an intervention that could be performed in a community setting including home, recreation centers, and the like. Studies published from 1981 through 2012 were included. A total of 29 studies were identified that met inclusion criteria, representing 2499 participants (1470 in exercise interventions and 979 in control interventions). The majority of studies (20 of 29, or 69 %) targeted individuals with fibromyalgia, 5 targeted OA, 2 RA, and 1 each included participants with either RA or lupus or OA and RA (Fig. 13.2). The median length of the intervention periods was 16 weeks, with a median of 3 sessions per week, and a median of 30 min per session. For the majority of studies in which the information was included, the intensity of training was classified as moderate. Drop-out from exercise groups ranged from 0 to 50 %, with a mean dropout rate of 16.2 ± 12.5 %; dropout from control groups was similar, ranging from 0 to 46 %, mean 14.1 ± 13.3 %. Interventions were both supervised and unsupervised, and took place in a variety of settings. Overall, the interventions demonstrated statistically and clinically significant reductions in depressive symptoms (SMD −0.42, 95 % CI −0.58 to −0.26), an effect similar to those found in general population studies. Because no clear evidence was found for a dose–response relationship, the authors suggest that the CDC physical activity guidelines are an appropriate starting point for activity recommendations.
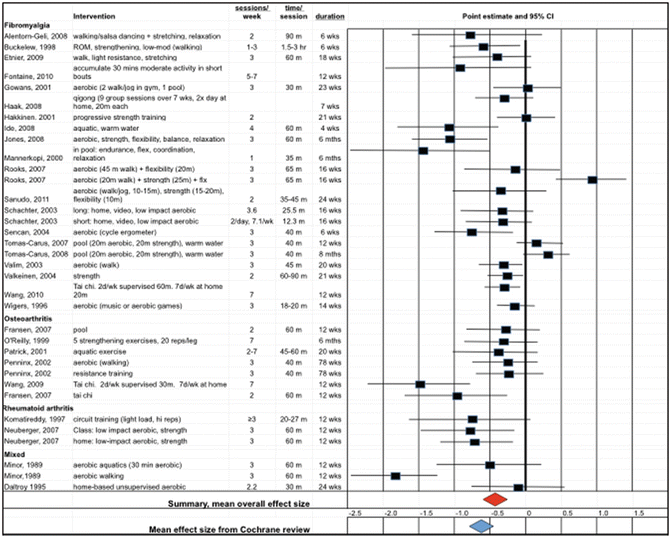

Fig. 13.2
Adapted from Kelley et al. (2015). The figure shows the point estimates (boxes) and 95 % confidence intervals (lines) for the reduction in depression conferred by the exercise intervention. The overall estimate of effect is shown by the red diamond. For comparison, the overall effect of exercise programs on depression in the general population, as estimated by Cooney et al. (2013), is shown as the blue diamond
Why Might Physical Activity Influence Depression?
Interventions have provided evidence that physical activity may reduce depressive symptoms in rheumatic diseases indirectly through reduced pain and better function. More recent evidence suggests that activity may also affect depression through mechanisms that are especially relevant to rheumatic conditions, based on the increasing recognition that depression is a pro-inflammatory state. Physical activity can directly lower systemic inflammation. Individuals who are more active also tend to have lower levels of adipose tissue and to report better sleep, both of which are also linked to lower levels of systemic inflammation and have well-established links with depression. Figure 13.3 illustrates potential pathways through which physical activity might influence depression. These pathways are interrelated—for example, pain can disturb sleep or increase fatigue, obesity increases systemic inflammation—so that physical activity may work through more than one pathway or may create synergistic effects.
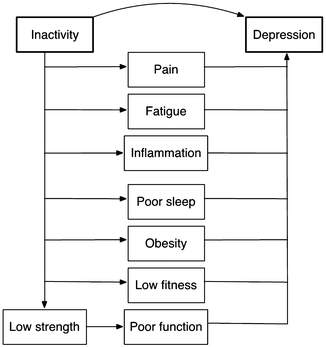

Fig. 13.3
Potential pathways through which physical inactivity might influence depression
There are additional effects of physical activity that are relevant to depression, such as effects on neurotransmitters in the brain and endorphins. These will not be discussed in this chapter because the effects are less specific to rheumatic conditions, but the reader is directed to recent reviews by Eyre and Prakash (Eyre, Papps, & Baune, 2013; Prakash, Voss, Erickson, & Kramer, 2015).
Pain. Higher levels of pain are consistently associated with a greater risk of depression (Goesling, Clauw, & Hassett, 2013; Kroenke et al., 2011). Previous exercise interventions among adults with arthritis have generally shown reductions in pain (Baillet et al., 2010; Conn, Hafdahl, Minor, & Nielsen, 2008). In fact, exercise had effects comparable to cognitive behavioral therapy in improving global health assessments among patients with chronic widespread pain (McBeth et al., 2012). The effects of exercise on pain seem to occur regardless of the type of exercise or activity. For example, muscle strengthening with or without aerobic exercise was found to be effective for pain relief in knee OA (Tanaka, Ozawa, Kito, & Moriyama, 2013), and consistent participation in the Arthritis Foundation’s People with Arthritis Can Exercise (PACE) and Walk with Ease programs resulted in reductions in pain (Callahan et al., 2008, 2011). In fibromyalgia , accumulating at least 30 min of lifestyle physical activity through the day are associated with improvements in pain (Fontaine, Conn, & Clauw, 2010), and people with fibromyalgia who are physically active appear more able to modulate pain (Ellingson, Shields, Stegner, & Cook, 2012; McLoughlin, Stegner, & Cook, 2011).
The reasons that exercise affects pain are less clear. There have been suggestions that exercise increases the production of endorphins, which inhibit the transmission of pain. There is also evidence that exercise may modify central pain processing (Walsh & McWilliams, 2014), and that the intensity of activity may influence pain sensitivity (Andrzejewski, Kassolik, Bzozowski, & Cymer, 2010). In one study, meeting the CDC physical activity recommendations was associated with a reduction in experimentally tested pain sensitivity (Ellingson, Colbert, & Cook, 2012). Only actual levels of physical activity (i.e., activity measured with an actigraph), as opposed to self-reported activity, showed an association, and the strongest association, with lower pain thresholds was noted with accumulation of vigorous activity.
Function. Function may be one especially potent pathway through which activity affects mood. Williamson demonstrated the impact of activity restriction on development of depression (Williamson, 2000; Williamson & Schulz, 1992, 1995). Among individuals with a range of chronic illnesses, exercise was shown to be most effective in reducing depressive symptoms when it also improved functional status (Herring et al., 2012).
In rheumatology, research has demonstrated that declines in functioning are strong predictors of subsequent onset of depression (Katz & Yelin, 1995, 2001). The declines in function that appear most important to the development of depression are losses of discretionary activities that are valued by individuals, particularly the loss of recreational and leisure activities and social interactions (Katz & Yelin, 2001). In contrast, the type of functioning usually measured in rheumatology, a lower level of functioning, is less predictive of the onset of depression (Fig. 13.4).
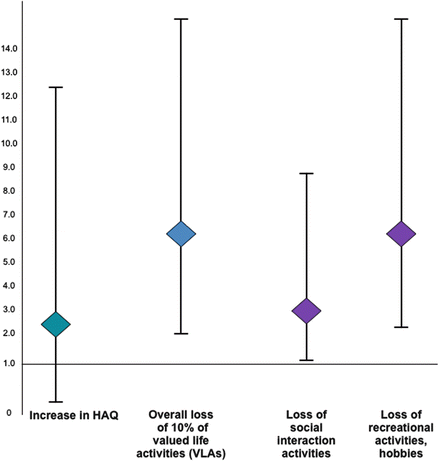

Fig. 13.4
Relationship of functional decline, measured by the Health Assessment Questionnaire (HAQ) , and valued life activity (VLA) loss with subsequent onset of depression. Valued life activities (VLAs) are the wide array of activities that are important to people. They range from self care to household tasks, family care, and work, to social and recreational activities (Katz, Morris, & Yelin, 2006). The VLA Disability scale asks respondents to rate the amount of difficulty they have in 26 specific activities. A short version of the VLA has also been developed (Katz et al., 2011). The Health Assessment Questionnaire (HAQ) was developed to measure function in rheumatoid arthritis (Fries, Spitz, Kraines, & Holman, 1980). It collects information on difficulty in actions such as standing up from a chair, cutting one’s meat, walking on flat ground, arising from a toilet, reaching above one’s head, and opening doors. This study examined losses of function and VLAs from Time 1 to Time 2, and the subsequent development of depression at Time 3 (Katz & Yelin, 2001). Loss of 10 % of VLAs was associated with an odds (95 % CI) of developing depression of 5.87 (2.10, 16.08). Losses in two specific domains also had significant elevated odds of subsequent development of depression: social interaction (OR 3.07, 1.04–8.89) and recreational activities and hobbies (OR 6.13, 2.04–18.82). In contrast, functional decline as measured by the HAQ was not a significant predictor of new depression
Physical activity can reduce functional limitations and reduce the risk of onset and progression of disability. Using data from the OAI, Dunlop demonstrated that greater time in light-intensity physical activity, measured by accelerometer, reduced the risk of disability onset and progression (Dunlop et al., 2014). Additional data from OAI showed that being less sedentary was associated with better function (Lee et al., 2015). Individuals who participated in the Arthritis Foundation’s PACE and Walk with Ease programs experienced improvements in function (Callahan et al., 2008, 2011). Higher levels of physical activity have been linked to greater improvements in functioning in rheumatoid arthritis, fibromyalgia, and ankylosing spondylitis (Baillet et al., 2010; Bennell & Hinman, 2011; Brophy, Cooksey, et al., 2013; Conn et al., 2008; Fontaine et al., 2010; Iversen et al., 2012; Kaleth, Saha, Jensen, Slaven, & Ang, 2013). Conversely, lack of exercise or low physical activity has been associated with increased disability and loss of muscle mass (Minor, 2004).
Fatigue and sleep disturbance. Fatigue and sleep disturbances may be both symptoms and predictors of depression. Fatigue is almost universally experienced by individuals with RA (Nikolaus, Bode, Taal, & van de Laar, 2013), and high levels of fatigue have been found in other rheumatic conditions, including osteoarthritis, systemic lupus erythematosus, psoriatic arthritis, ankylosing spondylitis, and fibromyalgia (Bergman et al., 2009; Brophy, Davies, et al., 2013; Clauw, 2014; Kim, Luedtke, Vincent, Thompson, & Oh, 2012; Murphy, Alexander, Levoska, & Smith, 2013; Sterling et al., 2014; Walsh et al., 2014; Wolfe, Hawley, & Wilson, 1996). Only a handful of studies have examined the impact of exercise on fatigue in RA, and in those, exercise training appeared to improve fatigue levels (Bilberg, Ahlmén, & Mannerkorpi, 2005; Hakkinen, Sokka, Leitsalmi, Kautianinen, & Hannonen, 2003; Neill, Belan, & Ried, 2006; Neuberger et al., 2007; Rongen-van Dartel et al., 2014). A meta-analysis of non-pharmacological interventions for RA fatigue concluded that exercise interventions appear to be effective in reducing fatigue in RA, with effect sizes slightly smaller than those of a well-designed cognitive behavioral program (~0.5 compared to 0.6–0.7) (Cramp et al., 2013). Physical activity or exercise interventions have been identified as effective interventions for fibromyalgia symptoms, including fatigue (Kaleth et al., 2014).
Self-reported sleep disturbances are also common in rheumatic conditions (Abad, Sarinas, & Guilleminault, 2008; Clauw, 2014; Luyster, Chasens, Wasko, & Dunbar-Jacob, 2011; Nicassio et al., 2012; Palagini et al., 2014; Reading et al., 2009; Roehrs et al., 2013; Taylor-Gjevre, Gjevre, Nair, Skomro, & Lim, 2010; Taylor-Gjevre, Nair, & Gjevre, 2013; Treharne et al., 2007). In the general population, higher levels of physical activity are linked to better sleep quality. Among a group of older women, higher levels of moderate–vigorous physical activity were associated with less sleep fragmentation and better sleep efficiency, even after adjusting for age, education, body mass index (BMI), depressive symptoms, and arthritis (Lambiasse, Gabriel, Kuller, & Matthews, 2013), and exercise interventions show improvements in sleep quality among depressed individuals (Rethorst et al., 2013).
In RA, Nicassio and Wallston found a cross-sectional association between sleep and depression (Nicassio & Wallston, 1992). Longitudinally, pain appeared to exacerbate sleep problems, which, in turn, increased depressive symptoms. Irwin and colleagues examined the impact of a partial night sleep deprivation, a proxy for a common form of sleep disturbance, on mood and RA symptoms (Irwin et al., 2012). Results indicated that such sleep disturbance induced significant changes in depressive symptoms (as well as pain) after a single night. An interventional study found a significant improvement in self-reported sleep quality after a 12-week exercise program among individuals with RA (Durcan, Wilson, & Cunnane, 2014).
Inflammation. Many rheumatic conditions are characterized by inflammatory states. For example, elevated levels of a number of inflammatory biomarkers, including tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6), are often noted in SLE and RA and are considered indicators of increased disease activity (Arend, 2001; Froneck & Horowitz, 2002). Likewise, depression is increasingly recognized as a pro-inflammatory state. Evidence includes observations that individuals with inflammatory diseases have elevated rates of depression, and significant portions of individuals with depression exhibit elevations in inflammatory factors, including C-reactive protein (CRP), IL-6, and TNF-α (Alexopoulos & Morimoto, 2011; Hamer, Batty, Marmot, Singh-Manoux, & Kivimäki, 2011; Maes, 2011; Matheny et al., 2011; Pasco et al., 2010; Prather, Rabinovitz, Pollock, & Lotrich, 2009). Increases in inflammatory factors often precede increases in depressive symptoms, and “cytokine-induced depression” is well documented in the literature (Dantzer, O’Connor, Freund, Johnson, & Kelley, 2008; Prather et al., 2009). Some recent studies have suggested that depression may be the causal factor for inflammation (Copeland, Shanahan, Worthman, Angold, & Costello, 2012; Shaffer et al., 2011; Stewart, Rand, Mudoon, & Kamarck, 2009), but the bulk of the research literature suggests the reverse—that inflammation increases the risk of depression (Krisknadas & Cavanagh, 2012; Leonard, 2010; Lotrich, El-Gabalawy, Guenther, & Ware, 2011; Miller, Maletic, & Raison, 2009; Raedler, 2011; Raison, Capuron, & Miller, 2006; Raison & Miller, 2011).
While acute, unaccustomed exercise may put a strain on the body, chronic physical activity improves immune function (Huang, Zourdos, Jo, & Ormsbee, 2013). Regular physical activity decreases inflammatory biomarkers, including CRP, IL-6, and TNF-α (Abramson & Vaccarino, 2002; Autenrieth et al., 2009; Colbert et al., 2004; Eyre et al., 2013; Ford, 2002; Lavie, Church, Milani, & Earnest, 2011; Reuben, Judd-Hamilton, Harris, & Seeman, 2003; Shanely et al., 2013). Sedentary behavior has been linked in recent studies with elevations in systemic inflammation biomarkers such as CRP, IL-6, and TNF-α (Allison, Jensky, Marshal, Bertoni, & Cushman, 2012; Yates et al., 2012). Benatti and Pedersen provide a comprehensive review of the evidence linking physical activity and systemic inflammation in rheumatic diseases (Benatti & Pedersen, 2015), highlighting the effects of physical activity on improvements in function and physical capacity and body composition (i.e., obesity), in addition to direct improvement in inflammatory biomarker profiles.
Obesity. Both obesity and physical inactivity have been linked to numerous negative health effects, including depression, in the general population (Herring et al., 2012; Luppino et al., 2010; Shelton & Miller, 2010, 2011; Simon et al., 2006; Teychenne et al., 2010a, 2010b). Adipose tissue is a known source of proinflammatory cytokines, including TNF-α and IL-6 (Coppack, 2001; Giles et al., 2008; Visser, Bouter, McQuillan, Wener, & Harris, 1999). Obesity, particularly abdominal obesity, is strongly associated with concurrent and incident depression (de Wit et al., 2010; Luppino et al., 2010; Rivenes, Harvey, & Mykletun, 2009; Vogelzangs et al., 2010, 2011; Zhao et al., 2009). There has even recently been suggestion that adipose tissue may be a causal pathway for the inflammation-depression link (Shelton & Miller, 2011). Regular physical activity generally decreases adipose tissue.
Obesity is common among individuals with rheumatic diseases. Rates approaching 50 % have been found in cohorts with RA and lupus (Katz et al., 2011, 2013), reported rates in fibromyalgia range from 33 to 45 % (Kim, Luedtke, Vincent, Thompson, & Oh, 2012; Segura-Jimenez et al., 2015), and elevated rates are seen in OA (Sturner, Gunther, & Brenner, 2000). Obesity has received little attention as a predictor or risk factor of depression in rheumatic diseases. Observational studies have found associations between obesity and depression in fibromyalgia (Aparicio et al., 2011; Kim et al., 2012). A recent study examined obesity as a risk factor for the development of depression among a group of approximately 500 women with lupus (Katz et al., 2014). Possible and probable depression were estimated using validated SLE-specific cut points on the CESD (20 and 24, respectively). Obesity was defined using a validated lupus-specific BMI2 cut point (BMI ≥ 26.8). Mean follow-up time was 9 years (range 2–11 years). In the analysis for possible depression, 31.6 % of those who were not obese became depressed, compared to 48.1 % of those who were obese (Table 13.1). In the analysis of probable depression, 25.9 % of those not obese became depressed compared to 42.6 % of those who were obese. After adjustment for covariates, obesity conferred an increased risk of depression of more than 50 %. Using the standard BMI cut point for obesity (BMI ≥ 30) yielded similar results.
Table 13.1
Risk of depression onset associated with obesity among women with SLE
Possible depression (CESD ≥20) | Probable depression (CESD ≥24) | |
|---|---|---|
Total n for analysis | 471 | 515 |
Obese at baseline | 38.9 % | 39.2 % |
Became depressed | ||
Not obese | 31.6 % (91) | 25.9 % (81) |
Obese* | 48.1 % (88) | 42.6 % (86) |
Multivariate HR (95 % CI) for obesity† | 1.56 (1.15, 2.11) | 1.69 (1.23, 2.30) |
Physical Activity and Well-Being
Psychological well-being is not just the absence of depression, however; it also includes the presence of positive affect. Broadly, there is a wealth of literature supporting the links between physical activity and positive mood, vigor, general well-being (Asztalos, De Bourdeaudhuij, & Cardon, 2010; Elavsky & McAuley, 2007). Additional psychosocial effects of exercise include distraction from negative thoughts, enhancement of self-esteem, and provision of a sense of mastery or self-efficacy (Eyre et al., 2013). Except for self-efficacy, exercise interventions in rheumatic diseases have not measured positive affect.
A variety of exercise interventions in rheumatic diseases have shown improvements in self-efficacy, which is linked to a number of positive effects including adherence to medications and other health recommendations, health behaviors, ratings of symptoms such as pain and joint stiffness, and function. Interventions showing effects on self-efficacy have included walking interventions and a variety of community-based programs combining strength, flexibility, balance, and aerobic conditions (Callahan et al., 2008, 2011; Levy et al., 2012; Schlenk, Lias, Sereika, Dunbar-Jacob, & Kwoh, 2011).
Qualitative studies offer insight into the impact of physical activity on well-being. Individuals with RA who had participated in an exercise program found that the program changed their self-image and led to improvements in overall well-being and feeling more energetic (Demmelmaier, Lindkvist, Nordgren, & Opava, 2015). They reported being now able to participate in social and recreational activities with friends and families in a way that was not possible prior to engaging in the exercise program. In addition, changes in perceptions of exercise were also reported—it was viewed as fun and rewarding. In another qualitative study, exercise was viewed as a means to resist disability and stay healthy, experience sensations of well-being, and engage in social participation on par with non-arthritis populations (Loeppenthin et al., 2014).
Physical Activity and Cognitive Function
Almost all of the literature on physical activity and cognitive function comes from outside rheumatology, often from studies of older adults. Because of the strong and consistent relationships found in those studies, and because cognitive dysfunction is increasingly recognized in rheumatic diseases, this topic is included here.
Cognitive Impairment in Rheumatic Conditions
Individuals with SLE are at significant risk of cognitive impairment (Ainiala, Loukkola, Peltola, Korpela, & Hietaharju, 2001; Brey et al., 2002; Denburg, Carbotte, & Denburg, 1997; Hanly, Fisk, McCurdy, Fougere, & Douglas, 2005; Kozora, Hanly, Lapteva, & Filley, 2008; Kozora, Thompson, West, & Kotzin, 1996; Petri et al., 2008; Unterman et al., 2011), and evidence is mounting that individuals with RA may also have elevated risk of cognitive dysfunction (Antonchak, Saoudian, Khan, Brunner, & Luggen, 2011; Appenzeller, Bertolo, & Costallat, 2004; Bartolini et al., 2002; Hanly et al., 2005; Shin, Julian, Katz, & Wallhagen, 2011). Examples of the prevalence of cognitive impairment in these two conditions may be drawn from studies by Julian (Julian et al., 2012) and Shin (Demmelmaier et al., 2015). Both studies used a neuropsychological battery spanning a range of cognitive domains and defined impairment on each domain as performance below −1 standard deviation of population normative data. For lupus, impairment ranged from 6 % (semantic fluency) to 30 % (visuospatial learning), and for RA, from 8 % (working memory) to 30 % (visuospatial learning). Using a summary definition of cognitive dysfunction (≥/3 indices impaired), 25 % of SLE patients, and 10 % of RA patients, met criteria for prevalent cognitive dysfunction. Cognitive dysfunction is also highly prevalent in fibromyalgia. Between 50 and 80 % of individuals with fibromyalgia have impairments in memory, attention, and/or executive function (Bertolucci & de Oliveira, 2013), and “fibro fog,” a constellation of cognitive issues including mental slowness, memory loss, and lack of clarity, is often cited as a symptom (Glass, 2009; Kravitz & Katz, 2015).
Physical Activity and Cognitive Impairment in Population Studies
Inactivity is linked to cognitive impairment in the general population (Angevaren, Aufdemkampe, Verhaar, Aleman, & Vanhees, 2008; Weuve et al., 2004; Yaffe, Barnes, Nevitt, Lui, & Covinsky, 2001; Zhu et al., 2014), and, conversely, higher levels of physical activity and fitness are associated with better cognitive function, particularly executive function3 (Baker et al., 2010; Colcombe & Kramer, 2003; Etgen et al., 2010; Middleton et al., 2011; Weuve et al., 2004; Yaffe et al., 2009). Data from the Coronary Artery Risk Development in Young Adults study (CARDIA) examined the impact of cardiorespiratory fitness, which can be viewed as the result of physical activity, on cognitive function 25 years after assessment of fitness, when participants were 43–55 years of age. Results indicated that compared to the lowest quartile of fitness, performance on each cognitive test was 21–34 % better in the highest fitness quartile. Effects were noted specifically for verbal memory and psychomotor speed (Zhu et al., 2014).
In reviewing physical activity and cognitive decline among older non-demented adults, Sofi concluded that individuals who performed high levels of physical activity gained significant protection against cognitive decline (HR 0.62, 95 % CI 0.54–0.70) (Sofi et al., 2011). The effect of low-to-moderate activity was only slightly less (HR 0.65, 95 % CI 0.57–0.75). Effects were slightly greater when longer follow-up periods were examined. Angevaren’s Cochrane review concluded that exercise interventions resulted in improved cardiorespiratory fitness, which coincided with improvements in cognitive functioning among older adults without known cognitive impairment (Angevaren, Aufdemkampe, Verhaar, Aleman, & Vanhees, 2008). Another meta-analysis of aerobic exercise interventions and cognitive performance found modest improvements in attention and processing speed, executive functioning, and memory (Smith et al., 2010). While the effects of physical activity on cognitive function of older adults seems clear, there is less evidence for younger adults due to paucity of studies (Prakash et al., 2015). Like sedentary behavior and depression, there also seems to be a circular relationship between inactivity and cognitive function. In a study of over 4000 older adults, inactivity led to declines in executive function, but the impact of poor executive functioning on later physical activity was even stronger (Daly, McMinn, & Allan, 2015).
Investigations into the mechanisms for physical activity’s effect on cognitive function are relatively recent and appear to show structural effects on the brain from physical activity. Physical activity has been shown to be protective of structural volume in the prefrontal cortex at 21-year follow-up (Rovio et al., 2010). Participation in physical activity also appears to be linked to a lower volume of brain white matter changes of the type considered a primary mechanism of age-related cognitive decline (Burzynska et al., 2014). The authors of that study speculated that the effect on white matter was due to the beneficial effects of physical activity on blood vessel integrity and elasticity. A more comprehensive review on this topic can be found in Prakash et al. (2015).
Physical Activity and Cognitive Impairment in Rheumatic Conditions
Little work has explored the potential link between physical activity and cognitive impairment in rheumatology. Inactivity was significantly and independently associated with executive function in women with lupus (OR 9.4 [1.7, 52.8]), even after controlling for age, race, education, disease activity, oral glucocorticoid use, obesity, and depression (Katz et al., 2012) (Fig. 13.5). Kozora reported a significant inverse relationship between cognitive impairment and 6-minute walk distance in lupus, a measure of functional cardiorespiratory fitness (Kozora et al., 2015).
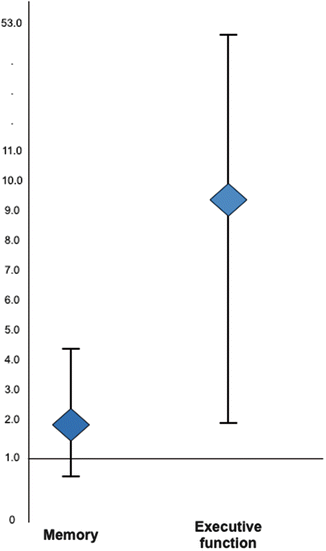

Fig. 13.5
Physical inactivity and risk of cognitive impairment . From Katz et al. (2012) Defining inactivity as <600 metabolic equivalent minutes per week, inactivity was associated with an significant elevated risk of impairment in executive function among women with systemic lupus erythematosus (Odds ratio = 9.4 [95 % confidence interval 1.7–52.8]). Inactivity was also associated with an elevated, but not statistically significant risk of impairment in memory function (OR = 1.8 [0.7, 4.4]). Activity was ascertained using the self-reported International Physical Activity Questionnaire (IPAQ) (Brown, Trost, Bauman, Mummery, & Owen, 2004; Craig et al., 2003). The definition of inactivity used was that specified in IPAQ scoring as “low activity”
Examining interventional studies, participants in a 6-week walking program based on Walk with Ease reported significant improvement in their ability to concentrate (Nyrop et al., 2011). There is some evidence that physical activity interventions improve cognitive function in fibromyalgia (Bertolucci & de Oliveira, 2013). Warm water exercise 3 times a week for 16 weeks demonstrated improvements in cognitive function among women with fibromyalgia (Munguia-Izquierdo & Legaz-Arrese, 2007), and an 18-week physical activity program produced improvements in self-reported cognitive function (Etnier et al., 2009).
Interventions and implementation
Barriers
In spite of the evidence supporting the benefits of physical activity, most studies show that individuals with arthritis are inactive. Some reasons for inactivity are similar to those expressed by the general population. For example, among the factors associated with inactivity in the QUEST-RA study , many are also associated with inactivity in the general population, such as female sex, older age, and less education (http://www.cdc.gov/physicalactivity/data/facts.html). Disease-specific barriers may also exist. Among individuals with RA, greater age, BMI, disease activity, and radiographic joint damage were associated with lower levels of physical activity (van der Goes et al., 2014). Fatigue and pain levels may also hamper participation in activity.
Psychological and perceptual barriers may hamper uptake of physical activity by individuals with arthritis, as well. Among a group of individuals with RA, 65 % of excess inactivity was accounted for by lack of strong motivation and lack of strong positive beliefs related to benefits of physical activity (Lee et al., 2012). A qualitative study found that individuals felt physical activity positively influenced health and well-being, but respondents also thought that activity might be a possible cause of arthritis (Kaptein et al., 2013). This group also discussed the impact of “role overload” in affecting decisions not to participate in activity. Patients have reported concerns about exercise causing harm to joints, not knowing what exercises to do, and not wanting to exercise because joints hurt (Law, Markland, Jones, Maddison, & Thom, 2013). Inactive individuals have been found to have negative disease-related outcome expectancies that were more distressing and that they believed were more likely to occur (Gyurcsik, Cary, Sessford, Flora, & Brawley, 2015). These issues are overlaid on barriers to activity caused by depression in general, such as loss of interest in activities and difficulty initiating activities.
Perhaps most importantly, individuals with rheumatic disease may not be getting the message from their physicians or other health care providers that physical activity is beneficial (Do, Hootman, Helmick, & Brady, 2011). Having a recommendation from a health professional may be the factor most strongly associated with engaging in physical activity or exercise (O’Donnell et al., 2013). This lack of advocacy for physical activity is not unique to rheumatology. An analysis of the 2011–2012 NHANES data found that over 50 % of adults who were completely sedentary had not been told by a health care profession to increase their exercise (Loprinzi & Beets, 2014).
Physicians and other providers may not have adequate information to guide their patients. In a survey from the Netherlands, rheumatologists, clinical nurse specialists, and physical therapists believed public health recommendations for moderate-intensity physical activity were attainable for persons with RA and that physical activity was an important health goal for RA, but most did not feel competent in offering advice about physical activity (Hurkmans et al., 2011). An additional barrier may be the advice individuals receive for how to manage disease symptoms. Pacing (e.g., taking breaks from activity) has traditionally been advocated as a way for people with arthritis to manage fatigue or pain (Murphy & Kratz, 2014). Yet one author writes, “Since patients with rheumatoid arthritis are already at risk for inactivity, further inactivation by activity pacing might potentially be harmful.”(Cuperus, Hoogeboom, Neijland, van den Ende, & Keijsers, 2012).
Practical Solutions
Many types of programs have been developed to help people with arthritis increase physical activity. There is no strong evidence to favor one type of program over another. The primary criterion for success may be whether an individual will initiate and maintain it.
Some people may prefer the structure of an organized exercise program. The Arthritis Foundation’s PACE and Walk with Ease programs are examples of such programs designed for people with arthritis. The Walk With Ease program (http://www.arthritis.org/we-can-help/community-programs/walk-with-ease) is offered in both a self-guided format or a group setting. Designed as a 6-week program to encourage people with arthritis to become and stay active, the group classes are structured around group walks. The home-based version includes online support such as video instruction, a message board and email alerts for meeting goals or milestones. Both versions encourage walking three times a week and include stretching and strengthening exercise, identifying barriers to exercise, and developing an individualized walking plan. The schedule of the WWE program would likely not meet the CDC activity recommendations without the addition of additional days or length of sessions, yet for inactive people, this type of program is a good place to start. Individuals who are depressed may benefit from the social contacts and support of group programs.
Participating in organized programs may be prohibitive for others due to logistic issues, cost, or other reasons. Walking may be the simplest way for many adults to increase their activity levels. Leisure walking, walking for transportation, or combination, are all effective means of increasing activity in older adults (Hekler, Castro, Buman, & King, 2012). Prescribing walking 5–7 days (vs. 3–5 days/week) a week at a moderate (vs. vigorous) pace, either in single or multiple sessions, may be the most effective way of increasing walking time (Williams, Matthews, Rutt, Napolitano, & Marcus, 2008). Importantly, walking interventions have shown statistically significant effects on depression (Robertson, Robertson, Jepson, & Maxwell, 2012), with effect sizes appearing to be within the same range as other exercise programs (SMD −0.86, 95 % CI −1.12, −0.61]). In contrast to other exercise programs, though, study completion rates of the 8 walking interventions reviewed were substantial higher, ranging from 75 to 100 %.
Use of pedometers is a simple means to measure and increase physical activity. A systematic review estimated that in a pedometer intervention of average duration (18 weeks), participants increased daily physical activity by almost 2500 steps more than controls by the end of the program (Bravata et al., 2007). Studies attempting to increase activity among older adults using pedometers and step diaries have found that individuals are able to use the devices easily and that compliance with pedometer and diary use is good (Bravata et al., 2007; Sugden et al., 2008). In an article reflecting on why pedometers work, Tudor-Locke points out that they are simple and accessible, low-literacy friendly, and the output is immediately understandable (Tudor-Locke & Lutes, 2009). Both self-monitoring by recording daily steps and having step goals appear to enhance the effectiveness of pedometer programs (Tudor-Locke & Lutes, 2009). In addition to the mechanical aspect of counting steps, many devices have online components to offer guidance in setting goals, activity tracking, and development of walking “buddies” or groups.
Physical activity guidelines for US adults call for 30 min per day of moderate-to-vigorous physical activity (MVPA) on at least 5 days per week (U.S. Department of Health and Human Services, 2008). Translating this to daily step counts yields a goal of approximately 8000 steps per day (Tudor-Locke, Leonardi, Johnson, Katzmarzyuk, & Church, 2011), about 3000 of which can be attributed to the 30-min exercise bout (assuming an average of 100 steps per minute) and the remaining 5000 to “background” daily activity (Tudor-Locke, Hatano, Pangrazi, & Kang, 2008). Research suggests that health benefits begin to accrue at 7500–9999 steps per day (Bravata et al., 2007; Jordan, Jurca, Tudor-Locke, Church, & Blair, 2005; Krum, Dessieux, Andrews, & Thompson, 2006; McKercher et al., 2009; Tudor-Locke et al., 2004, 2008). Increases of approximately 2800 steps on just 3 days a week have produced significant improvements in health outcomes (Church et al., 2009; Church, Earnest, Skinner, & Blair, 2007; Jordan et al., 2005; Martin, Church, Thompson, Earnest, & Blair, 2009). There is also evidence that <5000 steps per day is associated with health risk (Schmidt, Cleland, Shaw, Dwyer, & Venn, 2009).
Recognizing that the “background” levels of activity are likely to be lower for older adults and those with chronic illness, initial goals of 5500 steps per day have been suggested (Tudor-Locke et al., 2011). Even 5500 steps per day may be daunting for individuals who have been inactive. Setting lower initial targets, with the intention of gradual increases in steps over time, may seem more attainable, and lead to greater adherence to activity.
Walking-based interventions have been tested in rheumatic conditions to address symptoms, but not depression. In fibromyalgia , increasing physical activity through a “lifestyle physical activity” intervention (measured by steps/day) led to significant reductions in pain and perceived functional deficits after 12 weeks.(Fontaine et al., 2010) Another study of individuals with fibromyalgia demonstrated that increases of just 1000 steps per day predicted lower pain intensity, pain interference, and depressive symptoms (Kaleth et al., 2013). The author of that study recommended an accumulation of at least 5000 steps per day to reach clinically significant improvements, similar to the goal delineated by Tudor-Locke for adults with chronic illness.
A pedometer-based walking program for older adults with knee OA directed 10 % increases in steps every 4 weeks over 12 weeks and led to improved function, although depression was not measured (Talbot, Gaines, Huynh, & Metter, 2003). Walking ≥6000 steps day was found to protect against developing functional limitations in people with or at risk of knee OA (White et al., 2014). In fact, an analysis from the OAI suggests that if an intervention could move an individual from inactive to even insufficiently active and cost < $2900 over 2 years, it would be considered cost-effective based on quality-adjusted life years (QALYs) (Sun et al., 2014).
Table 13.2 provides some practical guidelines for introducing physical activity.
Table 13.2
Recommending physical activity and exercise for depression in people with arthritis
Type of activity | • There is no clear evidence on what type of exercise is the most beneficial for depression, although the best evidence supports aerobic activity. Aerobic activity can include activities such as walking, biking, or swimming |
• Walking may be the most approachable form of physical activity for currently inactive individuals. However, some individuals may prefer engagement with exercise groups, which can provide both structure and social interactions | |
• There is also evidence that resistance exercise may be beneficial for mood. CDC guidelines recommend 2 or more days per week of muscle-strengthening exercise | |
Frequency | • Frequency should be at least three times per week, although there is some evidence that for depression, the recommendation should be at least five times per week |
Duration | • Exercise bouts can be in segments of as little as 10 min. The goal is to accumulate a minimum of 150 min per week |
• New physical activity should be undertaken incrementally. Start with 10 min or so, and gradually increase | |
Intensity | • Activity should be of at least moderate intensity. Moderate intensity can be gauged using the “talk test.” If an individual can talk, but not sing, during the activity, the intensity can be considered of moderate intensity. In vigorous activity, an individual will not be able to say more than a few words without pausing for breath (http://www.cdc.gov/physicalactivity/everyone/measuring/index.html) |
Sit less | • “Sit less” is also an important message. Feehan and Westby point out that by standing up twice an hour during the 10 h most people are sitting during a day adds 40 min of light activity (Feehan & Westby, 2014). Other suggestions they provide are standing up and stretching during TV commercials or after a chapter in a book |
Other | • Physical activity is safe for people with rheumatic conditions
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|


