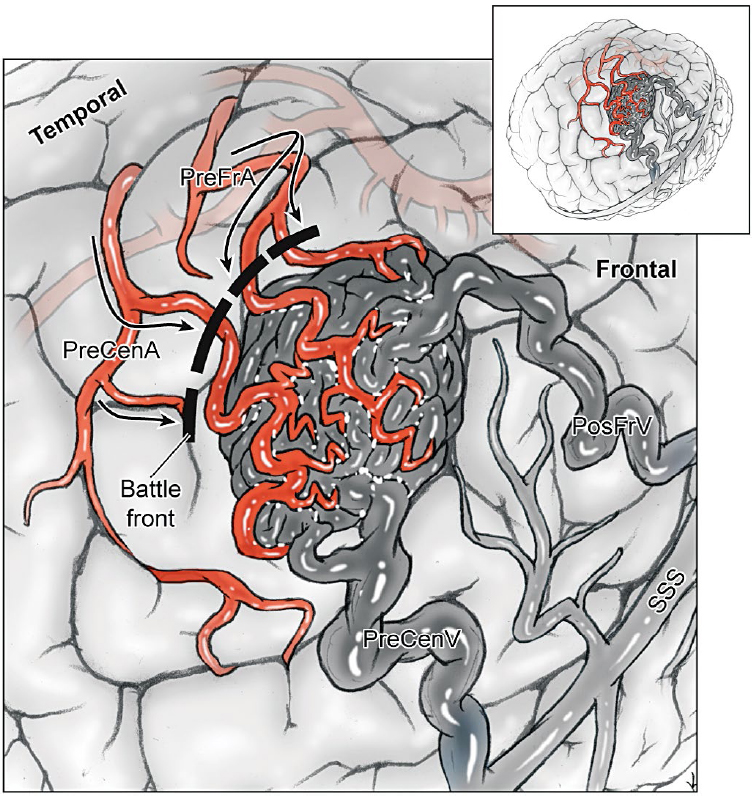
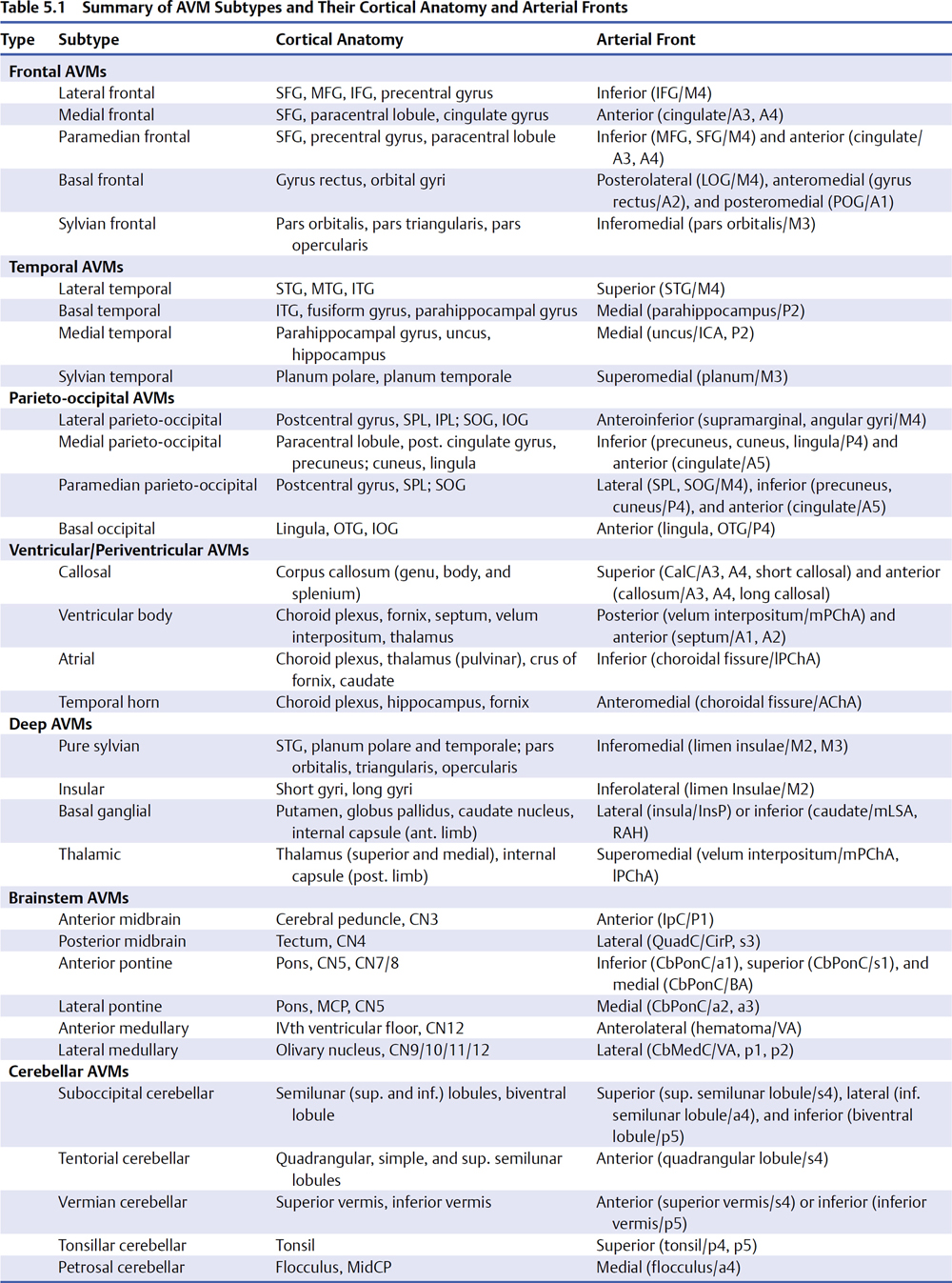
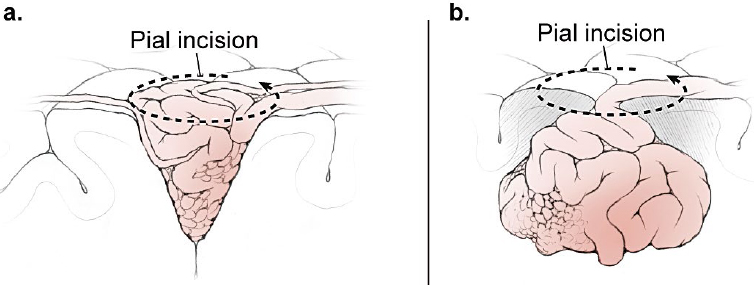
5 Pial Dissection Previous phases of dissection—arteriovenous malformation (AVM) exposure, subarachnoid approach, definition of draining veins, and the identification of feeding arteries—are mere saber-rattling. The AVM is surveyed but not disturbed. Pial dissection is the opening salvo and the start of the battle. Early engagement is directed at surface feeding arteries on the top of the box, making the easy strikes without major effort. Each AVM subtype has specific pial sites called arterial fronts where these superficial inputs intersect the AVM, like a line of battle where armies clash (Fig. 5.1). An arterial front lies on a side of the AVM box, usually an edge on the top side, and can be characterized by the side of the AVM box (anterior, lateral, superior, etc.), the cortical anatomy, and the feeding arteries. As an example, the lateral temporal AVM is based on the temporal convexity and supplied mainly by middle cerebral artery (MCA) branches from the inferior trunk of its bifurcation, like middle and posterior temporal arteries. These cortical arteries emerge from the sylvian fissure and travel across the superior temporal gyrus to reach the AVM’s superior margin. Therefore, the lateral temporal AVM has a superior front of M4 arterial supply defined by the superior temporal gyrus. Arterial fronts are the initial points of attack, occluding significant input and weakening the AVM early on. Fig. 5.1 Each AVM subtype, like this lateral frontal AVM (inset), has specific pial sites or arterial fronts where superficial inputs intersect the AVM like a line of battle where armies clash, defined by the AVM side, cortical anatomy, and feeding arteries. Arterial fronts are the initial points of attack (bold dashed line). An arterial front can be defined for every AVM (Table 5.1), and these fronts inform the pial dissection. Most AVMs have one major arterial front, such as the lateral temporal AVM, but some have as many as three. The basal frontal AVM that has a lateral front of M4 MCA branches (orbitofrontal artery [OrbFrA]) arriving over the lateral orbital gyrus, a medial front of A2 anterior cerebral artery (ACA) branches (OrbFrA and frontopolar artery [FrPolA]) arriving over the gyrus rectus, and a posterior front of A1 ACA branches arriving over posterior orbital gyrus. An AVM like the lateral temporal AVM can have a major superior front and a minor inferior front of posterior cerebral artery (PCA) supply if situated low on the convexity in inferior temporal gyrus. Some AVMs have one front that receives inputs from different arteries, like the medial temporal AVM that is supplied by the internal carotid artery (ICA) (anterior choroidal artery [AChA] and posterior communicating artery [PCoA]) and PCA (hippocampal artery and posterior temporal artery). Arterial fronts situated on pial surfaces are advantageous for their ease of attack, but some AVMs have pial fronts that are only accessible later after parenchymal dissection, like the medial front of posterior temporal arteries (P2 PCA) associated with a basal temporal AVM. Other deep AVMs have only parenchymal fronts, like the lateral front of insular perforators and the inferior front of lateral lenticulostriate arteries associated with a basal ganglial AVM. Pia mater is an important protective barrier that defines the boundaries of the subarachnoid domain. Microsurgical technique is most elegant when it respects this layer, as with aneurysm and bypass surgery. However, pia transgressions are inevitable requirements of AVM surgery. The pia must be incised to initiate circumdissection into brain parenchyma and advance the resection. Intact pia reassures us that brain is preserved, whereas violated pia worries us that neurologic morbidity might ensue. Therefore, pia is the anatomic link to eloquence, and the pial incision forces the neurosurgeon to confront eloquence at this phase of dissection, even though eloquence is a property of brain tissue and becomes a major factor later during parenchymal dissection. On the one hand, eloquence is obvious, defined in the Spetzler-Martin grading system as motor cortex, somatosensory cortex, language cortex (Wernicke’s and Broca’s areas), visual cortex, hypothalamus, thalamus, internal capsule, brainstem (midbrain, pons, and medulla), cerebellar peduncles, and deep cerebellar nuclei. On the other hand, eloquence is vague. Where exactly does visual cortex end peripherally in the occipital lobe? What about the eloquence of tracts like the optic radiations, whose disruption in temporal white matter produces a deficit similar to that in visual cortex? Does an AVM adjacent to the motor strip have the same eloquence as an AVM in the motor strip? Do pial AVMs in the brainstem and sylvian fissure have the same eloquence as a brainstem and sylvian AVM in parenchyma? Should the areas described by Spetzler and Martin as having more subtle neurologic function, including medial temporal lobe structures in the dominant hemisphere, nondominant parietal lobe, corpus callosum, cingulate gyrus, and basal ganglia, be treated differently from areas that are clearly non-eloquent, like the anterior frontal and temporal lobes and cerebellar cortex? Finally, can eloquence be graded accurately based on structural anatomy, when AVMs are known to dramatically alter functional anatomy, with shifts to an adjacent gyrus or relocations to the contralateral hemisphere? In short, eloquence is not so easy to judge intraoperatively. The uncertainty of eloquence is dealt with in three ways. First, functional magnetic resonance imaging (fMRI) can identify some areas of eloquence preoperatively. Deoxyhemoglobin has paramagnetic properties that enable blood flow to be measured at baseline and during the execution of a specific task using blood oxygen level dependent (BOLD) imaging. Blood flow delivers oxygen to active neurons, and subtraction paradigms between these two conditions couple flow and function both spatially and temporally to identify the brain regions involved in these tasks. Therefore, fMRI can localize motor and speech areas. However, unlike normal subjects, AVM patients have such deranged blood flow and adjacent arterioles are so chronically dysautoregulated that they respond poorly to tasks, and areas of functional activation appear larger than they should. Overestimations of eloquence discourage surgery. Other functional imaging techniques such as positron emission tomography (PET) and single photon emission computed tomography (SPECT) have similar problems. Magnetic source imaging (MSI) is not dependent on blood flow and instead measures neuronal activation by detecting magnetic flux associated with depolarization during synaptic transmission and intracellular ion currents. However, the imaging output from MSI is crude. Therefore, preoperative fMRI is generally unsatisfactory in localizing eloquence and is used to screen for major reorganization of eloquent cortex. Second, electrocortical stimulation mapping can be used intraoperatively to identify eloquent areas, specifically language and motor cortex. Ensuring patient comfort requires a coordinated team of neuroanesthesiologists, neurophysiologists, and neurosurgeons to control pain during the craniotomy, awaken the patient for language mapping, and conduct counting, naming, and reading tasks. Stimulation of language sites results in reproducible counting errors, speech arrest, or alexia. Motor and speech mapping is useful primarily for larger AVMs adjacent to these eloquent areas, below the cortical surface, or with diffuse borders. Localizing information helps protect eloquent cortex, select a dissection route to deep AVMs, perform corticectomy safely, and halt the dissection before complete resection to avoid morbidity. As important as this information is, intraoperative mapping is rarely used (3% in my experience). The AVM resection can be long, unpredictable, and uncomfortable for both the awake patient and neurosurgeon. Mapping encourages more conservative surgery, increasing our rate of incomplete resection from 2% overall to 25% with mapping, without clear proof that additional localizing data spared the patient a deficit. Therefore, mapping does not satisfactorily address the eloquence issue in most AVM patients either. Third, rather than defining eloquence radiologically or electrophysiologically, most neurosurgeons deal with eloquence by maintaining a close dissection distance from the AVM. Eloquence is assumed based on structural anatomy and protected during dissection with tight adherence to the AVM. We begin with a pial incision that closely circumscribes the AVM, and then we develop planes that hug the nidus and pull us in to minimize brain transgression. This tact is crude and works with compact AVMs, but clean planes rarely persist. Margins often become irregular or diffuse and the dissection migrates into parenchyma. In addition, bleeding from the nidus can push back the dissection. The comfort that comes from tightly adhering to the AVM is replaced with nagging concern about assumed eloquence. There is a dynamic between tight AVM adherence on the one hand and wide dissection distance on the other, between aggravating the AVM with the former and harming the patient with the latter. The end result is a constant, unrelenting tension that comes from uncertain eloquence, beginning with the pial incision and continuing throughout circumdissection. This is the psychology of eloquence that permeates AVM resections when our measures of eloquence are inadequate. In contrast to eloquent AVMs, non-eloquent AVMs have none of this tension or dynamic. A wider incision can be circumscribed in the pia to set a more generous course around the AVM that reduces the bleeding risk at no neurologic cost to the patient. Eloquent AVMs with both eloquent and non-eloquent sides can be managed similarly on their non-eloquent sides, but require tight adherence on their eloquent sides. Pial incisions should optimize the parallel exposure of the AVM’s sides, and a cone-shaped AVM based on the cortex requires a simple circular incision. However, a spherical AVM that widens beneath the cortex might require a pial incision beyond the AVM’s cortical margins (Fig. 5.2). Tight pial incisions create keyhole exposures with ledges of overlying brain and poorly visualized AVM margins that can be troublesome. When eloquence allows, wider pial incisions are safer for these deeper AVMs because they create funnelshaped exposure without ledges or blind spots.
 Arterial Fronts
Arterial Fronts
 Pial Transgression and Eloquence
Pial Transgression and Eloquence
 Dissection Distance
Dissection Distance
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree