Chapter 40 Pituitary Tumors
Diagnosis and Management
• Pituitary tumors present in various ways as a result of excess or deficient secretion of pituitary hormones or extrinsic compression on the pituitary stalk or adjacent structures. Approximately 75% of pituitary adenomas are functioning tumors; of these, half are prolactinomas, less than 25% secrete growth hormone (GH), and the rest secrete adrenocorticotropic hormone (ACTH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), or thyroid-stimulating hormone (TSH).
• Functioning tumors often present with symptoms due to hormone hypersecretion, and nonfunctioning tumors generally present with symptoms due to mass effect.
• Transsphenoidal adenomectomy (TSA) is the first line of treatment for nonfunctioning tumors. Medical therapy is the first line of treatment for prolactinomas. Other functioning adenomas generally require surgical resection, medical treatment, or radiation therapy. Surgery through the microscopic, extended transsphenoidal, or endoscopic route is safe and effective in experienced hands. Craniotomy may be required for a tumor extending beyond the sellar region.
• Patients with functioning pituitary adenomas require long-term follow-up to assess clinical and laboratory parameters. This is best done at a center that can provide a neurosurgeon, an endocrinologist, a radiation oncologist, and an ophthalmologist. Pituitary hormones should be regularly followed in all these patients; patients with residual tumor after treatment should be monitored with magnetic resonance imaging (MRI) scans, and patients with optic nerve compression require periodic formal visual field testing.
The Pituitary Gland
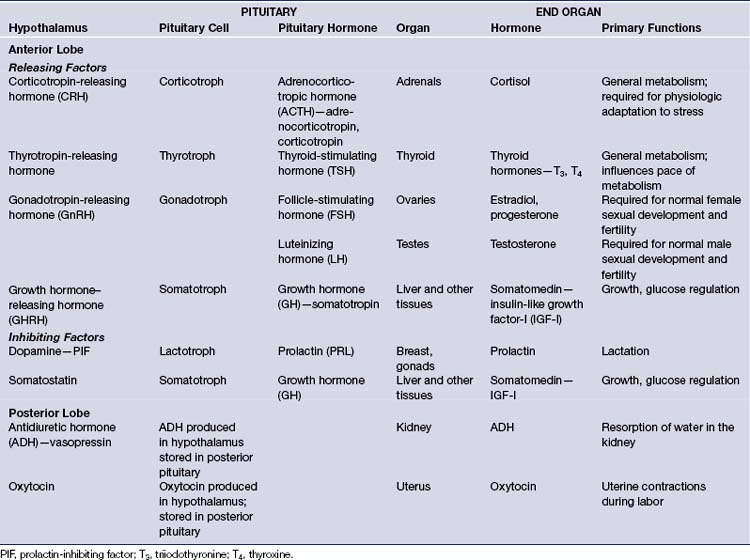
The pituitary gland regulates the function of numerous other glands, including the thyroid, adrenals, ovaries, and testes. It controls linear growth, lactation, and uterine contractions in labor, and it manages osmolality and intravascular fluid volume via resorption of water in the kidneys. It secretes eight peptide hormones, six from the anterior lobe and two from the posterior lobe (Table 40.1).
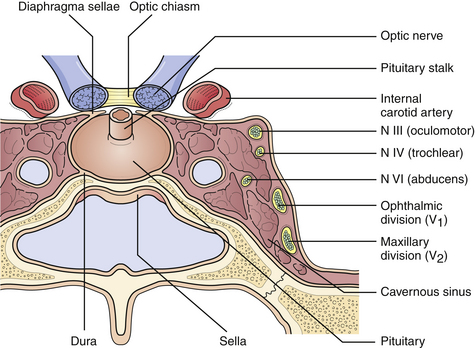
The pituitary lies in the sella turcica, a saddle-shaped concavity in the sphenoid bone. Its stalk, which contains the pituitary portal veins and neuronal processes, passes through the diaphragma sella, just above which pass the optic nerves (Fig. 40.1). The cavernous venous sinuses form the lateral borders of the sella, and contain within them the internal carotid arteries, cranial nerves III, IV, and VI, and the ophthalmic and maxillary divisions of cranial nerve V.
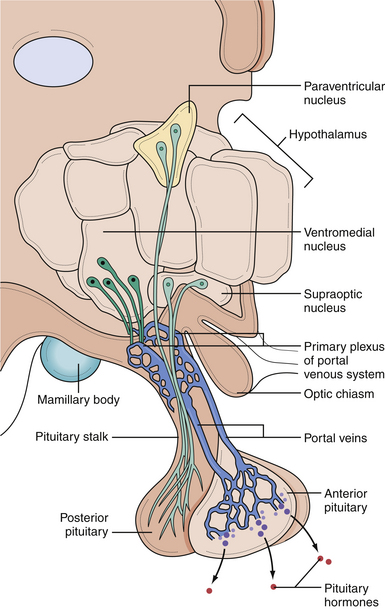
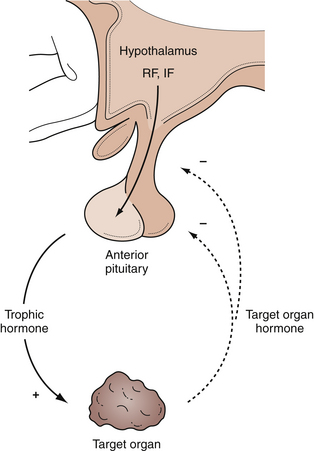
In the anterior lobe of the pituitary gland, known as the adenohypophysis, five distinct types of cells produce and secrete six different hormones. Lactotroph cells make prolactin (PRL), somatotroph cells produce growth hormone (GH), corticotrophs secrete adrenocorticotropic hormone (ACTH), thyrotrophs make thyroid-stimulating hormone (TSH), and gonadotrophs produce follicle-stimulating hormone (FSH) and luteinizing hormone (LH). The secretion of these hormones is regulated by the hypothalamus and by inhibitory feedback control by target organ hormone products (Figs. 40.2 and 40.3).
The hypothalamus secretes releasing factors to stimulate the production of pituitary hormones. Corticotropin-releasing factor (CRH), thyrotropin-releasing factor (TRH), and gonadotropin-releasing hormone (GnRH) positively regulate the production of ACTH, TSH, and the gonadotropins, LH and FSH. GH secretion is more complicated in that it receives both positive and negative hypothalamic manipulation, via GH-releasing hormone (GHRH) and somatostatin, respectively. Prolactin secretion is primarily inhibited by the hypothalamic release of dopamine, also known as prolactin-inhibiting factor. Some of these hypothalamic factors influence the production of more than one anterior pituitary hormone. For instance, TRH, which primarily stimulates TSH production, also has a positive effect on prolactin release.
Epidemiology
Pituitary adenomas have an annual incidence of 25 per 1 million people and account for nearly 10% of all surgically resected brain tumors, with nonfunctioning adenomas and prolactinomas being the most common pituitary tumors.1,2 Many of these tumors are subclinical and may never present during a patient’s lifetime; autopsy studies show an 11% to 27% incidence of occult microadenomas.3–6 Pituitary tumors are the third most common primary intracranial neoplasm, behind glioma and meningioma,7 and are more common among African Americans, in whom they account for more than 20% of central nervous system neoplasms.8
Microadenomas are most often found in women of childbearing age. Though studies in the 1970s appeared to demonstrate a higher incidence of adenomas among women than men, it is unclear if there is actually a higher prevalence among women or if the effect of a tumor on pituitary function and, therefore, reproduction leads to a higher rate of detection. Autopsy studies show no sex predominance.2 Men with pituitary tumors more often present with macroadenomas in their fifth and sixth decades of life.3
Classification
The major classifications of pituitary tumors are based on their size, secretory abilities, and histological type. Tumors with a diameter of less than 10 mm are microadenomas, and those greater than 10 mm are macroadenomas. Tumors greater than 4 cm are considered giant adenomas.
Functioning adenomas are generally classified by the hormones they secrete. Any of the multiple cell types within the pituitary can lead to a functioning pituitary tumor. Functioning tumors may secrete PRL, GH, ACTH, FSH, LH, or TSH. Nonfunctioning tumors do not secrete clinically relevant levels of hormone. However, even among nonfunctioning tumors, multiple different tumor types are found, including null cell, oncocytoma, silent gonadotropin or glycopeptide-secreting, silent corticotropin-secreting, and silent somatotropin-secreting.9
According to the World Health Organization (WHO), tumors with benign histological features are typical pituitary adenomas, but rare invasive tumors with an increased rate of mitosis and extensive p53 nuclear reactivity are classified as atypical adenomas. Pituitary carcinoma is very rare, constituting less than 0.2% of pituitary tumors, and the diagnosis is made only when distant metastases are found.10,11
Nonfunctioning Pituitary Adenomas
Nonfunctioning adenomas account for 30% of pituitary tumors.12 The term nonfunctioning reflects the fact that these tumors do not cause clinical hormone hypersecretion and so do not cause hypersecretory syndromes.13 These tumors are heterogeneous with multiple histopathological cell types. Though they do not cause signs of clinical hormone hypersecretion, histopathological evidence of hormone expression is evident in more than 40%.
Presentation
Nonfunctioning adenomas are benign lesions that are typically large upon presentation and manifest with symptoms of mass effect.14 Pressure on the pituitary gland can lead to decreased pituitary function, and nearly one third of these patients have hypopituitarism.15 Gonadotropins are generally the first hormones affected, then GH, followed by TSH and ACTH. Hormone dysfunction may lead to amenorrhea or hypogonadism and decreased libido, or to hypothyroidism with weight gain, depression, fatigue, and mental slowing. These changes develop insidiously as the tumor grows, so the patient may be unaware until the lesion is large.
Diagnosis
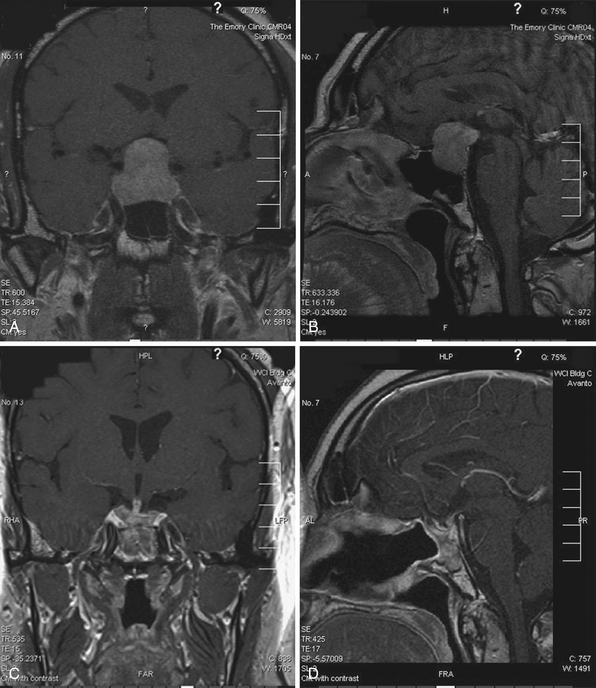
Plain radiographs may demonstrate an enlarged, round sella, and the sellar floor may appear doubled due to a thinned, asymmetrically worn lamina dura. High-resolution magnetic resonance imaging (MRI) with cuts through the sellar region is essential for surgical planning, as it will show the precise size and location of the lesion as well as its relationship with the chiasm, cavernous sinus, and other surrounding structures (Fig. 40.4), and a computed tomography (CT) scan will demonstrate the sphenoid sinus anatomy.
Treatment Options
Surgery
The first line of treatment for a nonfunctioning pituitary adenoma is transsphenoidal resection of the tumor, which provides immediate relief of mass effect and has a low rate of complications.16–19 This surgery is generally done as an elective procedure. An extended transsphenoidal approach may be needed when the tumor has reached beyond the sella and a transcranial approach or combined transsphenoidal-transcranial approach may be considered if there is significant supratentorial tumor extension.20,21
The goals of surgery are to eliminate mass effect from the pituitary and surrounding structures, to preserve or restore pituitary and visual function, to resect enough of the lesion to prevent recurrence, and to obtain tissue for histopathological analysis.22
Hermann Schloffer performed the first transsphenoidal resection of a pituitary tumor in 1907 and Harvey Cushing popularized it in the two decades afterward.23,24 Neurosurgeons have been trying to perfect the transsphenoidal adenomectomy (TSA) since. The standard methods in use today involve either an endonasal or sublabial approach to the sella.
Endonasal Transsphenoidal Approach
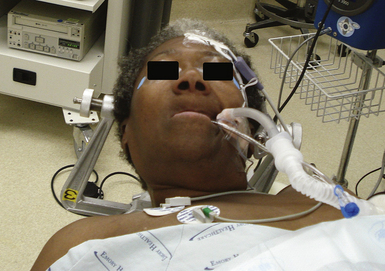
In the endonasal transsphenoidal approach, the patient’s head is placed in a Mayfield cranial fixation clamp. The body is placed supine with the neck somewhat extended and the head turned slightly toward the right to face the surgeon, permitting a good view through the nares (Fig. 40.5).
The operating microscope is brought into the field and the sphenoid sinus is opened with an osteotome and Kerrison rongeur. The opening is enlarged to allow visualization of the lateral portion of the sella. Some surgeons then obtain fluoroscopic or image-guided confirmation of the sella’s position; however, direct visualization is generally sufficient.
Endoscopy
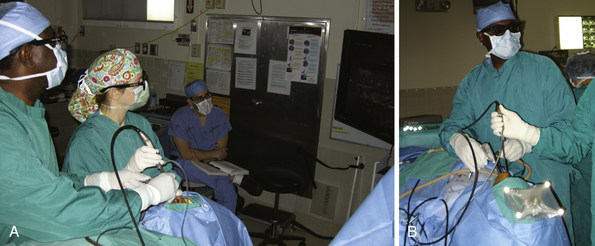
The lesion is removed in the same manner as with the microscopic approach. The endoscope provides a wide field of view and angled scopes permit enhanced inspection of the walls of the sella, as well as the suprasellar, retrosellar, and parasellar regions to search for residual tumor. Three-dimensional endoscopes have been introduced recently and permit a more realistic, nondistorted view of the regional anatomy than do the conventional two-dimensional endoscopes (Fig. 40.6).
After the lesion is removed, DuraForm is placed over the sella. Gelfoam is used to pack the sphenoid sinus, and NasoPore is laid over the bilateral sphenoethmoid recesses. If a CSF leak is present, a mucosal septal flap may be used to assist in closure. A speculum is not needed with this approach.25,26
Sublabial Transsphenoidal Approach
Pediatric patients with small nares or patients with large tumors may be treated via a sublabial TSA. The patient’s upper lip is retracted and a horizontal incision is made in the gingival mucosa. A pathway is followed to the maxilla and floor of the nasal cavity. A vertical incision is made, separating the nasal mucosa from the septum. The anterior septum is subluxed, the speculum is inserted, and the operating microscope or endoscope is brought into the field. The operation continues in a similar fashion to the endonasal approach just described.27
Neuronavigation
A TSA may be performed with frameless stereotactic neuronavigation to assist with large lesions that involve the carotid arteries or recurrent lesions where a prior operation has altered the normal anatomy.28,29 The operation is performed as just described; however, the surgeon is able to check his or her position at any point during the procedure to assess the proximity of surrounding structures or determine when he or she is approaching the limits of the tumor (Fig. 40.7).
Outcomes
Following operative decompression, vision generally improves and endocrine function recovers to a lesser extent, and surgical resection generally halts progressive loss of hormonal function. After TSA, visual field defects will improve in 70% to 89% of patients,30,31 will not change significantly in 7%, and will worsen in less than 4% of patients.32
Approximately 30% of patients with nonfunctioning adenomas have some degree of hypopituitarism prior to surgery.33 In one quarter of these patients, preoperative pituitary deficiencies will improve after surgery, although 10% of patients have some postoperative worsening of hormone function.8 Oral hormone replacement is generally sufficient for those patients whose pituitary deficiencies worsen or do not improve.
Complications from transsphenoidal surgery include intracranial hemorrhage, carotid artery injury, ischemic stroke, visual impairment, CSF leak, nasal septal perforation, and epistaxis. The risk of stroke or death is less than 1%, the risk of visual loss is less than 2%, and the risk of CSF rhinorrhea is less than 4%.34
Diabetes insipidus (DI) and the syndrome of inappropriate antidiuretic hormone secretion (SIADH) are common but transient postoperative complications after transsphenoidal surgery. Nearly 18% of patients will develop DI, though this is often temporary.34 It is imperative to closely monitor fluid balance, serum sodium levels, and urine specific gravity. In a small number of patients, hyponatremia can occur a week or more after the surgery.
Following resection of a giant macroadenoma, when it is likely that some residual tumor may remain, a rare but potentially fatal complication is postoperative apoplexy. This complication must be closely monitored for. In one study of 134 surgically resected giant adenomas, four patients had fatal postoperative pituitary apoplexy.35
The mortality rate for patients undergoing transsphenoidal surgery is very low, approximately 0.5%. Among giant macroadenomas, the mortality rate is approximately 1%. After successful surgical resection, 10% to 20% of tumors are reported to recur within 6 years.30,31 At 10-year follow-up, more than 80% of patients who underwent transsphenoidal resection of a nonfunctioning pituitary adenoma are alive and disease free.8
Medical Therapy
Medical therapy is available for functioning tumors, but there are no available effective drug regimens for nonfunctioning pituitary adenomas. Multiple medical therapies have been tested. Dopamine agonists lead to a small reduction in tumor size in less than 10% of patients, and octreotide has improved visual deficits among some patients with macroadenomas.30,31 However, most patients with nonfunctioning pituitary tumors will gain no clinical or biochemical benefit from medical therapies.
Radiation Therapy
Radiation therapy may be used in patients with recurrent or residual tumor, or in patients who cannot tolerate surgery. Radiotherapy controls tumor growth in 80% to 98% of nonfunctioning tumors.36
Conventional radiotherapy calls for fractionated doses of 1.6 to 2 Gy four or five times per week for 5 to 6 weeks, for a maximum dose of 45 to 50 Gy.9 Tumors respond slowly, with benefits delayed for a year or more.
Stereotactic radiosurgery (SRS) uses only a single session to deliver focused radiation to the lesion with less radiation to surrounding structures. Several forms of SRS are available, including Gamma Knife surgery (GKS), linear accelerator (linac) radiation, and CyberKnife surgery. There is also fractionated stereotactic radiotherapy and proton beam therapy. SRS is generally able to use a higher radiation dose per fraction than is conventional radiation, and usually results in earlier endocrine control. It is not risk-free, however.
The major concern from SRS is radiation damage to the visual pathways, but this can be decreased by limiting the radiation dose to the optic chiasm to less than 10 Gy.37,38 Patients with adenomas closer than 2 to 5 mm to the optic chiasm or larger than 30 mm in diameter are generally not candidates for SRS, though they may undergo fractionated stereotactic radiotherapy or conventional radiation.39 The neuronal and vascular structures in the cavernous sinus are less radiosensitive, so an ablative dose may be administered to tumors with lateral invasion or impingement on the cranial nerves. This allows SRS to function as an adjuvant to surgical resection in patients with tumors that have invaded the cavernous sinus.
As with conventional radiotherapy, hormone deficiencies are the most common side effect with an incidence of 13% to 56%.40–43 The risks of radiation-induced second neoplasm and neuropsychiatric changes are lower with SRS than with conventional radiotherapy. Other side effects are rare. Long-term risk for radiation necrosis is approximately 0.2%. Optic neuropathy occurs in 1.7%, vascular changes in 6.3%, neuropsychological changes in 0.7%, and radiation-induced secondary malignancies in 0.8%.44 All forms of radiation therapy have delayed benefit, though SRS induces remission more rapidly than does fractionated radiotherapy.42,45
Follow-up
Routine postoperative evaluation with MRI and CT should be delayed for at least 4 to 6 weeks after surgery because of the difficulty analyzing the tumor region in the setting of recent surgical changes. Mass effect seen in the early postoperative period usually resolves and can be followed with serial MRI.46
Functioning Pituitary Adenomas
The majority of pituitary tumors are functioning adenomas. Of these, PRL-secreting tumors, or prolactinomas, account for 40% to 60%, while GH-secreting tumors make up 15% to 25%.47 Corticotropin-secreting adenomas represent about 5% of functioning adenomas, and gonadotropin- and thyrotropin-secreting tumors account for less than 1%. Neurohypophyseal tumors are very rare.
Prolactinoma
Prolactin Physiology
Lactotrophs secrete prolactin and are unique in that normal cells can proliferate during adulthood. PRL interacts with receptors in the gonads and acts on breast tissue to initiate and maintain lactation. Hypothalamic moderation of PRL secretion occurs by the release of dopamine into the portal circulation from nerve processes that originate in the arcuate nucleus of the hypothalamus (Fig. 40.8). PRL release is increased by TRH, vasoactive intestinal peptide (VIP), GnRH, peptide histidine methionine, opiates, and estrogen. Pharmacological doses of TRH lead to a rapid release of PRL; however, the physiological role of TRH in PRL production is unclear.
During pregnancy, estrogen stimulates lactotroph hyperplasia and hyperprolactinemia but blocks the action of PRL on the breast, inhibiting lactation until after delivery. Within 4 to 6 months after delivery, basal PRL levels return to normal.
Incidence
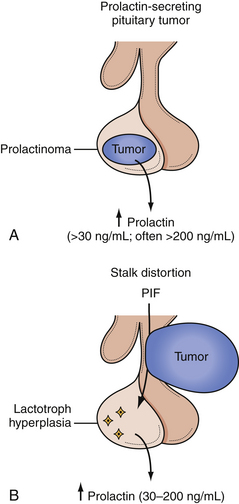
Prolactinomas are second only to nonfunctioning adenomas in overall incidence, composing about 30% of all pituitary tumors and more than half of functional pituitary tumors. Hyperprolactinemia from a prolactin-secreting adenoma causes hypogonadism and galactorrhea.48–50 Women with hyperprolactinemia usually present with amenorrhea, galactorrhea, diminished libido, and infertility. Gonadal dysfunction and decreased estrogen secretion may lead to osteoporosis. In men, hyperprolactinemia manifests as diminished libido, impotence, gynecomastia, or infertility due to a decreased sperm count. Adolescents may present with delay of sexual development. In children and postmenopausal women, signs of hypogonadism may be absent.50
In autopsy studies, prolactinomas are found in equal numbers of men and women, but women are four times more likely to become symptomatic. Because they develop symptoms sooner, women more often present with smaller tumors, and men generally present with larger tumors and mass effect. Approximately 5% of women with primary amenorrhea and 52% with secondary amenorrhea not due to pregnancy have a prolactinoma, as do about 2% of men with impotence.51
Evaluation
Normal PRL levels are approximately 5 to 20 ng/mL in men and 5 to 25 ng/mL in nonpregnant women. Any sellar mass can compress the pituitary stalk and interrupt dopaminergic inhibition of PRL. This leads to a “stalk effect,” which results in mildly elevated PRL levels, generally 20 to 150 ng/mL, but should not be confused with a true prolactinoma. Serum PRL levels tend to correlate with the size of the prolactinoma, so that microadenomas generally lead to serum prolactin levels of 100 to 250 ng/mL, and macroadenomas may lead to a serum PRL well above 200 ng/mL. Invasive adenomas or giant adenomas may cause a serum PRL to be several thousand to 100,000 ng/mL.50,52,53 A PRL level more than 200 ng/mL is nearly pathognomonic for a prolactinoma. In patients with amenorrhea, however, pregnancy must also be excluded, as it is associated with PRL levels of 100 to 250 ng/mL by the third trimester. A PRL level of less than 2 ng/mL is generally associated with hypopituitarism, though this may be due to PRL-lowering medications.
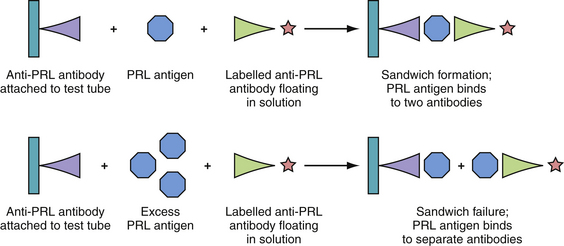
One must be suspicious of the “hook effect,” if prolactin values are very low in the presence of a suspected giant prolactinoma.54,55 If the serum PRL level is extremely high, the amount of prolactin-antigen may saturate the antibodies in the radioimmunoassay, failing to form the antigen plus two-antibody “sandwich” complexes required for accurate measurement, and thus leading to a falsely low value (Fig. 40.9). If a prolactinoma is suspected, the PRL level should be tested with serial dilutions to determine an accurate PRL value.52,56
Dopamine inhibits PRL secretion, so any drug that decreases dopamine levels will increase serum PRL. These drugs include many antidepressants, such as tricyclics, monoamine oxidase inhibitors, and selective serotonin reuptake inhibitors, as well as methyldopa, reserpine, and verapamil.57 Other medications such as phenothiazines and metoclopramide block dopamine receptors, indirectly leading to increased PRL levels.57
Medical Treatment
Dopamine agonists are the treatment of choice for prolactinoma.58 These agents can normalize PRL levels within hours to days, shrink tumors, restore reproductive and sexual function, and allow patients to avoid the potential risks of surgery.
Current dopamine agonists, including bromocriptine, cabergoline, and pergolide, are synthetic derivatives of ergot alkaloids.59 Bromocriptine is begun orally once daily and increased over several weeks to multiple daily doses. It decreases tumor volume in approximately 85% of patients, and reduces PRL levels to normal in nearly 90%.50 Bromocriptine works very rapidly, often leading to a decrease in PRL within 1 to 2 hours. More than 10% of tumors are not sensitive to bromocriptine,60 and 5% to 10% of patients are intolerant of its gastrointestinal side effects, though intravaginal administration may lessen these symptoms.61
Cabergoline is more expensive than bromocriptine, but may be better tolerated, and is administered once or twice weekly.58 It normalizes PRL levels in up to 84% of patients.62 Studies of cabergoline in women of childbearing age are limited, but bromocriptine has been extensively studied and appears safe in pregnancy. Therefore, if pregnancy is desired, bromocriptine should be used,63 then discontinued during pregnancy unless symptomatic tumor enlargement occurs.
Medical treatment is generally continued chronically as cessation of either drug may result in recurrent hyperprolactinemia and tumor re-expansion.64 After initiation of medical therapy, MRI and visual examination should be repeated, and serum PRL levels should be monitored at least annually.65,66
One must be cautious with prolonged use of cabergoline and other ergot-derived dopamine agonists, as they may lead to an increased risk of pleural and pericardial fibrosing serositis and valvular heart disease when used in large doses, as they are in patients with Parkinson’s disease.67–69 Patients with prolactinoma receive significantly smaller doses of the drug than do patients receiving the drugs for parkinsonism, and it is unknown if the risk of symptomatic valvular heart disease exists at these low doses. Studies of echocardiograms in patients receiving cabergoline for hyperprolactinemia have reported conflicting results.70–74 There does not appear to be any statistical increase in incidence of clinically relevant or symptomatic cardiac regurgitation in patients treated with cabergoline. We recommend echocardiographic evaluation of patients who are receiving long-term, high-dose cabergoline.
Surgery
The effectiveness and safety of medical therapy have limited the need for surgery in most patients with prolactinoma. If a patient fails to respond to or is unable to tolerate the side effects of medical treatment, then surgery is recommended, as medication may be more effective after surgical debulking.75,76 Surgery is also suggested for patients who develop CSF leak while undergoing medical therapy or patients who have a dissociated response to drugs in which their prolactin level falls but the tumor does not shrink. TSA, as described previously, is the surgery of choice.34,77 An extended transsphenoidal approach may be needed if the tumor is beyond the sella, and craniotomy should be considered if there is significant extension.20,78
The higher the PRL level, the lower the chance of surgical cure. Patients with microprolactinomas and serum PRL level below 200 ng/mL have a greater than 90% chance of cure with TSA when performed by experienced pituitary surgeons at high-volume centers, and morbidity and mortality risks are less than 1%.34,75 However, patients with a preoperative PRL level above 200 ng/mL with large, invasive prolactinomas have a less than 41% surgical cure rate.76 In patients with giant or invasive prolactinomas, pretreatment with a dopamine agonist may improve the success of surgery; however, long-term pharmacotherapy can alter the tumor’s consistency and make surgery more challenging.79
Radiation Therapy
Radiation therapy is an option for patients who have failed surgery or medical therapy.80,81 Though there is a risk of hypopituitarism or damage to the optic nerves, radiation therapy is generally considered safe and effective. Radiotherapy may be combined with medical or surgical management and may be delivered as conventional external beam radiotherapy or SRS.
Conventional radiotherapy regimens use approximately 4500 cGy in 25 to 30 fractions.82 Tumor growth is controlled in 83% to 100% of patients, and reduction in tumor mass is achieved in 36% to 45% of patients.83 SRS may provide more rapid endocrine control while permitting the delivery of radiation in a single session with less exposure to surrounding normal tissue.
Dopamine agonists may provide a radioprotective effect on tumors, and preferably should be discontinued during radiosurgery.80 In a study of 164 patients with prolactinoma who underwent primary treatment with Gamma Knife, tumor growth was controlled in all but two patients, and biochemical cure was attained in more than half.81
Growth Hormone–Secreting Adenomas
Growth Hormone Physiology
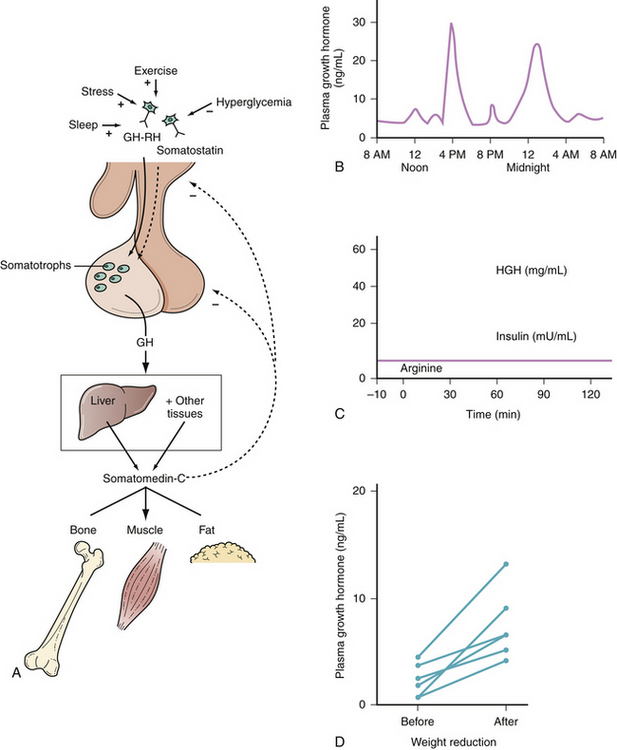
GH is required for normal human growth; it plays little role in the first year of life, but becomes very important during puberty. GH is required for normal linear growth and is secreted in pulses by the somatotroph cells. Its release is controlled by GHRH and somatostatin, which stimulate and inhibit release, respectively (Fig. 40.10). GH, in turn, stimulates the liver’s production of somatomedin-C, also known as insulin-like growth factor 1 (IGF-1). IGF-1 inhibits GH secretion at the hypothalamus, where it stimulates somatostatin release, and at the pituitary, where it suppresses GHRH-induced GH secretion.
Sleep, stress, exercise, and hypoglycemia increase the release of GH, while obesity, hyperglycemia, and excess glucocorticoids decrease it. GH is anabolic and increases the uptake of amino acids into tissue; conversely, a rise in amino acids increases GH release in a healthy individual. GH and IGF-1 levels are highest in children and young adults, then decrease with age in normal subjects. In normal subjects, serum GH levels are very low or undetectable for most of the day. GH has a half-life of 20 to 30 minutes and is secreted in short pulses, with two to seven peaks per day, leading to significant fluctuations in levels during the day. Some of these bursts are associated with meals, while others occur during the early stages of sleep. The half-life of IGF-1, on the other hand, is 2 to 18 hours, and serum levels are relatively stable. IGF-1 measurements therefore provide a more reliable indication of the exposure of the body to GH than do GH measurements.
Growth Hormone Excess: Acromegaly
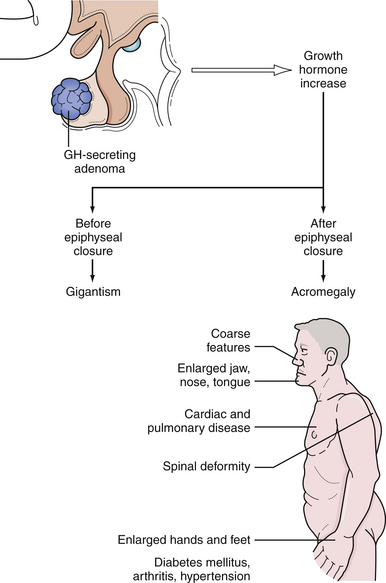
Excess GH secretion, which results in excess growth of the soft tissues, bony changes, and multiple biochemical changes, produces the syndromes of acromegaly in adults and gigantism in children who are affected before epiphyseal closure. Characteristics of acromegaly include coarse facial features with prognathism and malocclusion of the teeth, enlargement of the paranasal sinuses with frontal bossing, deepening of the voice, organomegaly, hyperhidrosis, acanthosis nigricans, enlargement of the hands and feet leading to an increase in ring, glove, and shoe size, and headache84–86 (Fig. 40.11). Insulin resistance can lead to diabetes mellitus. Tongue enlargement produces obstructive sleep apnea. Accumulation of excess soft tissue in the hands results in a wet, doughy handshake and in the feet it produces increased heel-pad thickness on radiographs. Patients with acromegaly also suffer from headache, proximal myopathy, osteoarthritis, carpal tunnel syndrome, cardiomegaly, and hypertension. Metabolic derangements lead to accelerated atherosclerosis and a shortened life expectancy as a result of cardiovascular, cerebrovascular, and respiratory causes. There is also a higher risk of developing other neoplasms, especially colon cancer.1,87 With appropriate reduction of GH levels, however, mortality risk decreases and can normalize.88
The average annual incidence of acromegaly is approximately 3.3 per million.88 Ninety-eight percent of patients with acromegaly harbor a GH-secreting pituitary adenoma.89 From 20% to 50% of these tumors also secrete PRL or other pituitary hormones.90 Rarely, acromegaly is the result of ectopic GH-producing tumors such as bronchial carcinoid or pancreatic islet cell tumors, a hypothalamic GHRH-releasing tumor, exogenous administration of GH for antiaging treatments, or familial syndromes such as multiple endocrine neoplasia I, McCune-Albright syndrome, or Carney complex.89
The disease is generally insidious and presents in the third to fourth decade, and both sexes are equally affected.91 The average patient with acromegaly has had symptoms for 8 to 10 years before the diagnosis is made, so the majority of GH-secreting adenomas are large at the time of diagnosis.88 Many compress the optic system and produce loss of visual acuity or bitemporal hemianopia by the time the tumor is recognized.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree