Pleasure
SEEKING PLEASURE
Voluntary behavior in animals is motivated by the avoidance of pain and the pursuit of pleasure. In this chapter, we will focus on the neuronal mechanisms that guide our choices toward stimuli that give a little reward to the brain.
Our existence—as individuals and as a species— is dependent on using the five senses to recognize and pursue actions necessary for survival. The motivation to pursue a beneficial act is driven in part by giving the brain a brief squirt of euphoria. This reward system has evolved over millions of years to enable an individual to sort through a variety of stimuli and choose the ones that are appropriate.
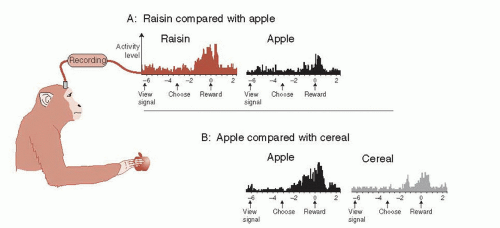
The orbital aspect of the prefrontal cortex (PFC) is known to play a critical role in goal-directed behavior. Figure 12.1 shows that the relative appeal for an item can be recognized in the brain even down to the level of a single neuron. In this study, electrodes placed in a single neuron in the orbitofrontal cortex registered different levels of activity based on the appeal of the food reward. A raisin generated the largest signal and was the most desired object; cereal was least active and least desired.
Many of our patients, family, and friends struggle with problems that they have created by pursuing the wrong rewards. Clearly, the addicted individual has lost control over his choices (more on this later), but what about the promiscuous
college student, the young adult who accumulates excessive debt on new credit cards, or the child who plays video games instead of doing homework? These people are choosing pleasurable activities that are not to their benefit and even harmful.
college student, the young adult who accumulates excessive debt on new credit cards, or the child who plays video games instead of doing homework? These people are choosing pleasurable activities that are not to their benefit and even harmful.
DISORDER MARITAL CONFLICT
Disparity in what brings joy is the major source of conflict in most relationships. Married couples frequently argue about sex, money, and how to spend leisure time. With each conflict, one partner wants to spend more time and money involved in some activity. For example, he wants to golf and buy a big boat for fishing; she wants to vacation with her family and fix up the house. He wants more sex; she wants more romance. It is ideal if his joy and her joy are in synch.
The problems are related to how our reward system is designed. We are built for acquiring rewards that were historically in short supply. Now with the extraordinary success of the human race and industrialized civilization, we are exposed to abundance beyond what our wiring has evolved to handle. Junk food, pornography, shopping malls, as well as alcohol, stimulants, and opioids usurp the mechanisms developed to enhance the survival of the hunter-gatherer in all of us.
Happiness
There is clinical evidence to suggest that one’s level of happiness remains remarkably fixed. For example, the person winning a lottery or the one suffering the loss of a limb tends to revert to their preexisting level of happiness after a period of euphoria or depression. It seems that happiness is hardwired and closely fluctuates around a genetic “set point” for each person. Unfortunately, there is surprisingly little neuroscience research on this topic.
ANATOMY OF REWARD
Pursuing a reward can be conceptualized as a ball rolling down the hill. Animals will gravitate toward the most enjoyable activity the way a ball rolls to the lowest point. It seemingly happens without effort. However, although we cannot visualize the force of gravity pulling on a moving ball, we continue to tease out the neuroanatomy of the mammalian reward system.
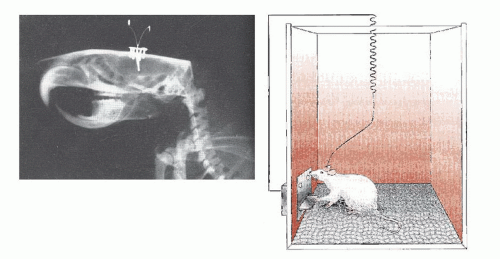
The initial studies came in the mid-fifties when Olds and Milner accidentally discovered that a rat would seek to continue stimulation from a thin electrode implanted in certain parts of his brain. Figure 12.2 shows the relative placement of an electrode in a rat’s skull and the apparatus that Olds and Milner used to document the animals’ efforts to stimulate themselves. Remarkably, the rats exceeded all expectations in what they were willing to sacrifice to receive stimulation. Depending on
where the electrode was placed, they would press the lever up to 5,000 times in an hour, chose stimulation over food even when starving, and cross an electrified grid for a chance to press the lever.
where the electrode was placed, they would press the lever up to 5,000 times in an hour, chose stimulation over food even when starving, and cross an electrified grid for a chance to press the lever.
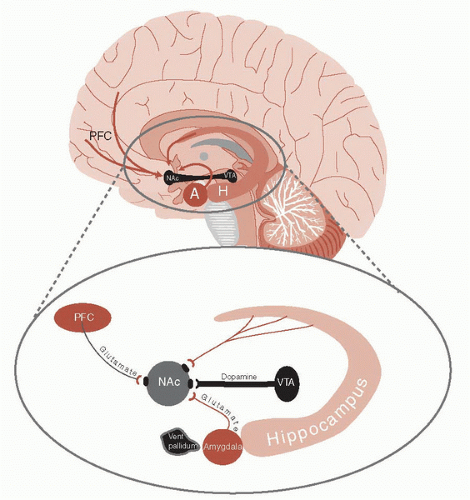
Fifty years of research has established that the mesolimbic dopamine (DA) systems, including the ventral tegmental area (VTA) and nucleus accumbens (NAc) (also called the ventral striatum), are the central structures of reward. These old but effective nuclei lie at the base of the brain (Figure 12.3) and are the structures that were being indirectly stimulated in the Olds and Milner experiments. The NAc and VTA receive signals from a multitude of sources—the most prominent of which are the PFC, amygdala, and hippocampus. It is significant that the input to the NAc and VTA originates from the areas involved in attention, executive decisions, and emotional memories— areas that help the brain negotiate the path to the reward.
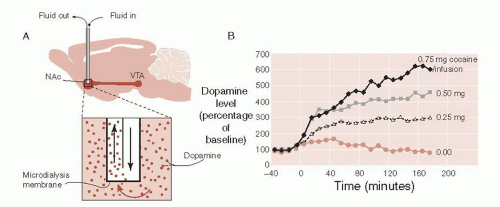
Converging lines of evidence have shown that DA is the primary neurotransmitter that modulates the reward system. Using an implanted microdialysis apparatus, Pettit and Justice were able to regularly sample DA concentration at the NAc. They found a correlation between the amounts of cocaine a rat would self-administer and the extracellular DA at the NAc (Figure 12.4). Alternatively, blocking the effect of cocaine either with a DA antagonist (e.g., haloperidol) or lesioning the dopaminergic cells in the NAc eliminated the self-administration and drug-seeking behavior.
Brain Imaging
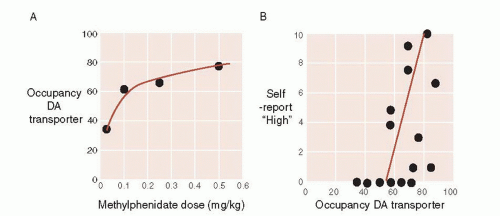
More recently, brain imaging studies with human volunteers have further established the link between DA and pleasure. Volkow administered IV methylphenidate (Ritalin) and established a correlation between the dose of the medication and the occupancy of the DA transporter (stimulants work in part by blocking the DA reuptake pump). Additionally by asking the participant how they felt, a correlation between feeling “high” and occupancy of the DA transporter was established (Figure 12.5).
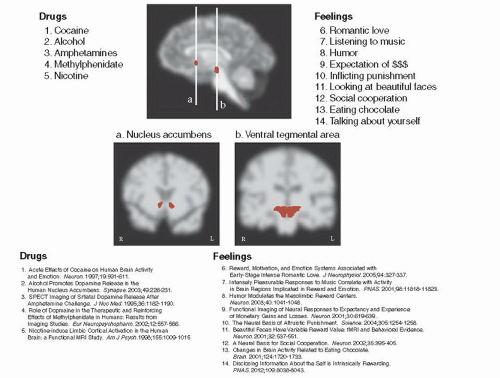
Creative studies with functional imaging scanners have shown that it is not just drugs of abuse (amphetamines, alcohol, nicotine, etc.) that result in enhanced DA at the NAc, but many pleasurable activities. Figure 12.6 gives examples of feelings (looking at beautiful faces, eating chocolate, revenge, etc.) as well as of drugs that have all demonstrated increased DA at the NAc and/or VTA in functional scans in humans.
Figure 12.6 shows only the results with humans. There are additional hedonic experiences that show similar findings with animals. For example, sexual behavior, violence, opioids, and marijuana all increase DA at the NAc in animals. Furthermore, we can speculate that other pleasurable behaviors— those that would be difficult to investigate in a scanner, such as shopping, gambling, extreme sports, and defeating ones arch enemy—may also deliver a dollop of DA to the NAc. The key point is this: Behaviors that people enjoy seem to precipitate an increase in DA produced at the NAc.
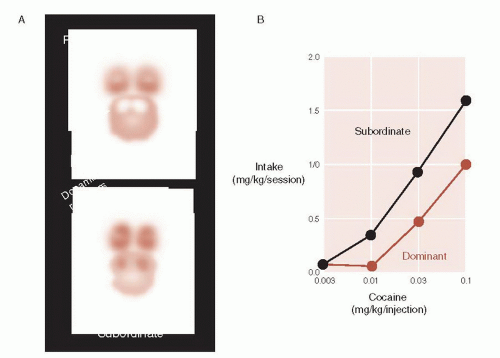
One particularly interesting study conducted at the primate laboratory at Wake Forest University looked at the effect of social rank on DA in monkeys. The monkeys were initially housed individually and scanned for DA D2 receptors, which were found to be similar for all monkeys. Next, they were housed together in groups of 4. After 3 months, the researchers determined who were the dominant and subordinate monkeys and
then re-scanned them. The results are shown in Figure 12.7. Note that the dominant monkey now has significantly greater DA D2 receptors in the striatum—an area that includes the NAc.
then re-scanned them. The results are shown in Figure 12.7. Note that the dominant monkey now has significantly greater DA D2 receptors in the striatum—an area that includes the NAc.
DISORDER COMPLICATED GRIEF
It is not uncommon to see patients who are experiencing prolonged and unabated grief of a loved one. Recent functional brain scans have shown persistent activation of the NAc in patients with such complicated grief: more activity in the NAc correlated with greater yearning when viewing a picture of the deceased. This report suggests that successful adaptation to loss is complicated by the joy of remembering—even when the memory is also painful.
The researchers also allowed the monkeys to self-administer different doses of cocaine. The graph on the right of Figure 12.7 shows that the dominant monkeys gave themselves less cocaine than the subordinates at different strengths of the drug. This suggests that the “good feelings” that come from being the alpha monkey buffer against seeking external sources of pleasure. This provides some insight into the correlation between increased substance abuse and lower socioeconomic status.
Pleasure versus Novelty
It is overly simplistic to call the NAc the pleasure center. Research with rodents has found that DA will increase at the NAc with adverse stimuli— for example, a foot shock. Likewise, addicts have reported that they have continued to seek their drug, although it is no longer pleasurable.
Some have suggested that the NAc determines the “wanting” and motivation to seek a reward—not the pleasurable experience itself. Volkow, director of the National Institute of Drug Abuse, prefers the term “saliency” to describe the function of DA at the NAc. By salience, she means new and unexpected stimuli that focus attention and motivate to seek more.
Di Chiara has studied the effect of various substances on DA release at the NAc. His group has shown that DA release is strongest for positive, novel reinforcements. Figure 12.8 shows the bump in DA at the NAc when a rat is given chocolate. However, when given the same amount of chocolate on the following day, the bump in DA is no longer significant.
Di Chiara has shown in other studies that habituation does not occur with cocaine. Day after day, rats will have a bump in DA at the NAc when given cocaine. Not only is the increase stronger in magnitude (up to 400% over baseline compared with 150% for chocolate) but also does not attenuate like the natural reinforcers. This may in part explain why cocaine is so addictive.
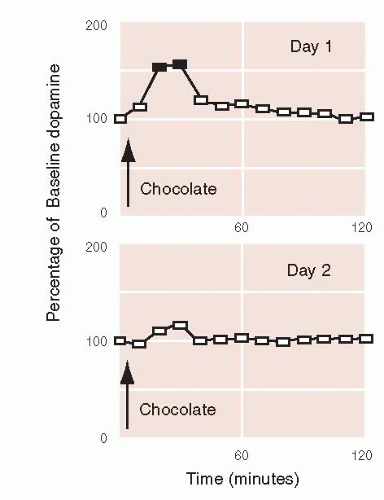 FIGURE 12.8 • Habituation. When first given chocolate, a rat shows a significant increase (▪) in its dopamine at the nucleus accumbens, which does not occur when given the same food on the following day. (Adapted from Bassareo V, De Luca MA, Di Chiara G. Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. J Neurosci. 2002;22(11):4709-4719.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|