Fig. 18.1
Chemical structure of short-chain ω-6 arachidonic acid (AA) and long-chain ω-6 linolenic acid (LA)
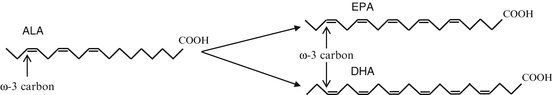
Fig. 18.2
Chemical structure of short-chain ω-3 α-linolenic acid (ALA) and long-chain ω-3s eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)
DHA and EPA contribute to phospholipid membrane fluidity, which allows for normal lipid and protein, receptor, channel, and transporter function within a cell membrane. When depleted, EPA/DHA are replaced by ω-6 resulting in stiffer membranes. DHA, found in large concentration in the cone and rod cells of the retina as well as the brain, is vital to neuronal health, growth, maturation, and function. Depletion of DHA in the CNS negatively impacts membrane structures, as well as the activity of membrane-bound proteins, receptors, and enzymes (Perica and Delas 2011). This may subsequently impair neurotransmitter binding, metabolism, and reuptake. Both EPA and DHA decrease pro-inflammatory effects in endothelial cells (increase nitric oxide production and improve arterial compliance), adhesion molecules (inhibit plaque formation within vessels), and sodium channels (reduce arrhythmias). Some early evidence suggested ω-3 may inhibit platelet aggregation by reducing thromboxane and inhibiting cyclooxygenase (COX) enzymes, however it was later deemed that supplementation with ω-3 did not adversely affect coagulation when taken at proper doses.
Both EPA and DHA can be synthesized endogenously from the essential fatty acid ALA, which must be derived entirely from dietary sources. The synthetic reaction converting ALA to EPA and DHA is very inefficient in humans (Perica and Delas 2011; Freeman and Rapaport 2011; Sanchez-Villegas et al. 2007; Yates et al. 2014; Goren and Tewksbury 2011; Shen et al. 2014). Diets rich in APA tend to increase concentrations of EPA but not DHA. Exogenously, ALA is found in plant sources, while EPA and DHA are found in marine animals and algae sources. Human bioavailability of PUFA is variable from marine algae yet high from fish. Therefore, human dietary intake of EPA and DHA from oily fish (herring, salmon, mackerel) is more effective at maintaining PUFA levels than relying on the inherently inefficient endogenous synthesis from dietary short-chain precursors. The long-chain ω-6 AA, like the long-chain ω-3, can be synthesized from ALA or acquired from dietary intake. These dietary fatty acids are then stored in membrane phospholipids and assist in modulation of the inflammatory response. It has been found that high levels of EPA and DHA in cellular phospholipids can decrease levels of AA, in turn decreasing the inflammatory response as both EPA and AA utilize the same metabolic enzymes. This may result in decreased production of eicosanoids, cytokines, and adhesion molecules by ω-6.
Inflammation and Fatty Acids
Acute inflammatory processes are adaptive and protective physiologic responses to infection and injury (Yates et al. 2014). At the site of local injury or infection, inflammatory cytokines (chemokines, interleukins, lymphokines, tumor necrosis factor) and histamine attach to vascular endothelial cell receptors, which in turn triggers the leukocyte adhesion cascade. Leukocytes within tissues trigger remodeling and cytotoxicity reactions, that in the setting of acute injury or infection are protective. Acute systemic inflammation stimulates the expression of acute phase proteins as well as the development of sickness behaviors. Systemic inflammation causes a decrease in appetite and concentration, as well as increased fatigue and irritability. In chronic inflammatory states however, leukocytes are continually captured from blood, infiltrating across affected endothelial cells into underlying tissue. This may result in tissue remodeling and subsequent damage. On a systemic level, chronic inflammation is associated with a host of potential pathological consequences including: fatigue and depressive symptoms, anorexia, muscle-coasting, obesity, insulin resistance, dyslipidemia, decreased androgen production, increased cortisol, decreased para-sympathetic tone, increased sympathetic tone, hypertension, inflammatory anemia, and osteopenia (Straub 2012).
Eicosanoids and docosanoids are molecules that modulate the immune system, inflammation, and platelet activity (Goren and Tewksbury 2011). Eicosanoids are derived from AA and EPA and docosanoids are derived from DHA. While AA-produced eicosanoids are critical for acute inflammation, ongoing or excessive production will lead to chronic inflammatory and subsequent tissue damage. This AA-mediated inflammatory cascade is modulated by the effects of EPA-produced anti-inflammatory or less potently inflammatory eicosanoids as well as anti-inflammatory DHA-derived docosanoids. Consequently the level of activity of inflammatory reactions is dependent upon the ω-3:ω-6 ratio.
Pro-inflammatory cytokines trigger the production and release of eicosanoids by stimulating PUFA release from membrane phospholipids (Shen et al. 2014). When released, the ω-6 AA is metabolized into pro-inflammatory eicosanoids in one of three ways: by COX to produce prostaglandins and thromboxanes; by lipooxygenases (LOX) to produce lipoxins, hydroxyeicosatetraenoic acids (HETE), and leukotrienes (LT); or by p450 epoxygenases to produce epoxyeicosantrienoic acids (EET) and HETEs. These eicosanoids subsequently generate the inflammatory symptoms of erythema, swelling, pain, and heat.
COX and LOX metabolize EPA and DHA into alternative eicosanoids, including specialized pro-resolving mediators (SPMs), called resolvins and protectins, which generate a less intense inflammatory response or have an outright anti-inflammatory action. SPMs block production of prostaglandins, leukotrienes and cytokines, inhibit neutrophil recruitment, and induce phagocytosis of apoptotic cells by macrophages. It was therefore believed that increasing dietary EPA/DHA would tilt the balance towards resolution of inflammation and improved tissue homeostasis by decreasing production of inflammatory eicosanoids and cytokines as well as increasing SPM synthesis.
ω-3 PUFA incorporated into cell phospholipid membranes increases membrane resistance and integrity of epithelial cells and block inflammatory cytokine action on those cells. They also inhibit synthesis of the ω-6 AA, further decreasing levels of pro-inflammatory eicosanoids. Despite the impacts of ω-3 on the inflammatory reactions described above, studies in humans have failed to demonstrate direct benefit from ω-3 dietary supplementation in inflammatory diseases. There may be indirect benefit of ω-3 supplementation from alterations in intestinal flora and downstream effects on microbiota-induced inflammatory reactions (Rosenblat et al. 2014; Yates et al. 2014; Shen et al. 2014).
Major Depressive Disorder
Initial epidemiologic studies suggested a correlation between increased fish consumption and lower rates of depression and suicide related behaviors (Murakami et al. 2010; Tanskanen et al. 2001; Timonen et al. 2004; Sublette et al. 2006). This led to a large number of investigations into the direct antidepressant action of ω-3 PUFA in patients with mood disorders. Antidepressant activity of ω-3 PUFA supplementation in Major Depressive Disorder (MDD) has been scrutinized in a wide variety of heterogeneous studies (Table 18.1). Only randomized controlled trials specifically in subjects with unipolar depression are included here. The largest trial of ω-3 PUFA in subjects with MDD is the SU.FOL.OM3 study by Adreeva et al. (2012). This study showed no significant benefit of ω-3 supplementation in a sample of 2,501 geriatric patients. There are five other large studies that include a total of 898 patients (Antypa et al. 2012; Carney et al. 2009; Grenyer et al. 2007; Lesperance et al. 2011; Rogers et al. 2008) and two smaller studies (Marangell et al. 2003; Mischoulon et al. 2009) with another 70 patients that found no benefit of ω-3 supplementation in MDD. These findings were contradicted by four mid-size trials (Mozaffari-Khosravi et al. 2013; Peet and Horrobin 2002; Sinn et al. 2012; Rizzo et al. 2012) and five small studies (da Silva et al. 2008; Gertsik et al. 2012; Jazayeri et al. 2008; Nemets et al. 2002, 2006), altogether including 406 patients that found significant decreases in depression severity with ω-3 supplementation. One study by Antypa et al. found conflicting results between the symptom measurement scales utilized; reporting no difference on the Beck Depression Inventory (BDI-II) or the Leiden Index of Depression Sensitivity-Revised (LEIDS-R) but significant decreases in depression severity scores on the profile of mood states (POMS) in response to ω-3 consumption. Most of the positive studies were augmentation studies, with patients remaining on antidepressant and psychotherapy regimens. Four of the positive studies were monotherapy designs, however one included only patients with mild to moderate depression (Mozaffari-Khosravi et al. 2013), a second was conducted with pediatric subjects (Nemets et al. 2006), and the remaining two were in geriatric subjects (Sinn et al. 2012; Rizzo et al. 2012). Therefore, there is limited evidence supporting ω-3 monotherapy or augmentation therapy to standard antidepressants in adult patients with severe MDD.
Table 18.1
ω-3 efficacy in major depressive disorder
Study | Population | N | Intervention | Duration (weeks) | Primary outcome measure | Results | Comments |
|---|---|---|---|---|---|---|---|
Unipolar depression RCTs | |||||||
Andreeva et al. (2012) | MDD, CHD, geriatric | 2,501 | Combination B-vit + EPA/DHA 0.6 g; EPA/DHA 0.6 g; B-vit; PLB | 156 and 260 | GDS | No difference; increase in males (GDS > 10) | Predominantly males |
Antypa et al. (2012) | MDD | 71 | EPA/DHA 2.3 g; PLB | 4 | POMS, BDI-II, LEIDS-R | No difference in BDI-II & LEIDS-R; decreased depression, tension, fatigue in POMS | Cognition & mood study |
Carney et al. (2009) | MDD, CHD | 122 | EPA/DHA 2 g + antidepressant; PLB + antidepressant | 10 | BDI-II, HDRS | No difference | Augmentation study |
da Silva et al. (2008) | MDD, PD | 29 | EPA/DHA 0.3 g; EPA/DHA + antidepressant; PLB; PLB + antidepressant | 12 | MADRS | Decreased depression in all EPA/DHA groups | Compares monotherapy vs. augmentation |
Gertsik et al. (2012) | MDD | 42 | EPA/DHA + citalopram; PLB + citalopram | 9 | HDRS | Decreased depression | Augmentation study; groups separated at week 4 |
Grenyer et al. (2007) | MDD | 83 | EPA/DHA 3 g; PLB | 16 | HDRS, BDI | No difference | Augmentation study; all subjects underwent weekly psychotherapy |
Jazayeri et al. (2008) | MDD | 48 | EPA 1 g; fluoxetine 20 mg; EPA 1 g + fluoxetine 20 mg | 8 | HDRS | Decreased depression with combination EPA/fluoxetine | Monotherapy and augmentation study; Monotherapies equivalent |
Lesperance et al. (2011) | MDD in MDE ≥4 weeks, ±anxiety | 432 | EPA/DHA 1.2 g; PLB | 4 | IDS-SR30 | No difference overall | 40 % maintained on antidepressants, 15 % maintained in psychotherapy; decreased depression in group without anxiety |
Marangell et al. (2003) | MDD | 35 | DHA 2 g; PLB | 6 | HDRS | No difference | |
Mischoulon et al. (2009) | MDD | 35 | EPA 1 g; PLB | 8 | HDRS | No difference | |
Mozaffari-Khosravi et al. (2013) | MDD | 81 | EPA 1 g; DHA 1 g; PLB | 12 | HDRS | Decreased depression with EPA only | Mild-moderate baseline MDD |
Nemets et al. (2002) | MDD | 20 | EPA + antidepressant; PLB + antidepressant | 4 | HDRS | Decreased depression | Augmentation study |
Nemets et al. (2006) | MDD, children | 20 | EPA/DHA 0.28–0.6 mg; PLB | 16 | CDRS | Decreased depression | Low placebo response rates |
Peet and Horrobin (2002) | MDD | 70 | EPA 1 g; EPA 2 g; EPA 4 g; PLB | 12 | HDRS | Decreased depression in 1 g | Augmentation study |
Rizzo et al. (2012) | MDD, geriatric females | 46 | EPA/DHA 2.5 g; PLB | 8 | GDS | Decreased depression | No change in immune markers |
Rogers et al. (2008) | MDD | 190 | EPA/DHA 1.5 g; PLB | 12 | DASS | No difference | |
Sinn et al. (2012) | MDD, geriatric | 50 | EPA 1.67 g; DHA 1.55 g; ω-6PUFA 2.2 g | 24 | GDS | Decreased depression with both EPA and DHA | Improvement in cognition also noted |
Cardiac patient studies on depression outcomes | |||||||
Bot et al. (2010) | DM type II | 25 | EPA 1 g + antidepressant; PLB + antidepressant | 12 | MADRS | No difference | Augmentation study |
Colangelo et al. (2009) | Young adult, CARDIA study | 3,317 | EPA; DHA; EPA/DHA | 20 year | CES-D | Decreased depression in females | No psych history |
Haberka et al. (2013) | Acute MI | 52 | EPA/DHA 1 g; PLB | 4 | BDI | Decreased depression | No psych history |
Zimmer et al. (2013) | Post-MI, OMEGA trial | 2,081 | EPA/DHA 0.84 g; PLB | 52 | BDI-II | No difference | Both monotherapy and adjunctive therapy; post hoc positive for efficacy |
Perinatal female studies on depression outcomes | |||||||
Doornbos et al. (2009) | Healthy, perinatal females | 182 | DHA 0.22 g; DHA/AA | 14–20 | EPDS | No difference | No psych history |
Freeman et al. (2006b) | Postpartum females | 16 | EPA/DHA 0.5 g, 1.5 g or 2.8 g; PLB | 8 | EPDS | Decreased depression | No psych history; No difference between doses of EPA/DHA |
Freeman et al. (2008) | MDD, perinatal females | 51 | EPA/DHA 1.9 g + psychotherapy; PLB + psychotherapy | 8 | EPDS, HDRS | No difference | Psychotherapy adjunct |
Krauss-Etschmann et al. (2007) | Healthy, perinatal females | 270 | EPA/DHA 0.65; PLB | 18 | HDRS | Decreased depression | Concomitant folic acid |
Llorente et al. (2003) | Postpartum females | 89 | DHA 0.2 mg | 12 | EPDS | No difference | No psych history |
Lucas et al. (2009) | Psychological distress ± MDE, females | 120 | EPA/DHA 1.2 g; PLB | 8 | HDRS | Decreased depression in women without MDE | No difference in MDE |
Marangell et al. (2004) | MDD, perinatal females | 7 | EPA/DHA 2.96 g; PLB | 12 | HDRS, EPDS | No difference | Euthymic upon study entry |
Mozurkewich et al. (2013) | Perinatal females, risk for depression or hx of depression | 126 | EPA-rich; DHA-rich; PLB | 40–48 | BDI | No difference | |
Su et al. (2008) | MDD, pregnant | 24 | EPA/DHA 3.4 g; PLB | 8 | HDRS | Decreased depression | Monotherapy, no antidepressants |
CVD and depressive disorders are intimately linked, and depressive symptoms are associated with a 2–2.5-fold increase in morbidity and mortality in CVD patients (Lett et al. 2008; Whooley et al. 2008). During investigations into ω-3 effectiveness in CVD, the question arose as to the impact of ω-3 supplementation on depressive symptoms in the setting of CVD. There are four studies that have examined the impact of ω-3 treatment on depression severity in patients with established CVD or cardiac risk factors. The largest study was a retrospective data analysis by Colangelo et al. of the coronary artery risk development in young adults (CARDIA) dataset of 3,317 young adults with no prior psychiatric history (Colangelo et al. 2009). At 10-year follow-up, women who received ω-3 PUFA had lower rates of depression than women who did not receive PUFA. Haberka et al. also found significantly lower rates of depressive symptoms in patients with recent myocardial infarction but no existing psychiatric history, who received ω-3 treatment after the index cardiac event (Haberka et al. 2013). These results suggest that ω-3 supplementation may have a protective effect against the development of mood disorders; however, they do not address the impact of ω-3 treatment on existing depressive symptoms. In the OMEGA study, 2,081 patients entered a depression sub-study after 12 months of EPA/DHA or placebo supplementation (Zimmer et al. 2013). Three hundred patients had mild depression and entry into the depression sub-study. In this analysis, ω-3 supplementation was ineffective as a monotherapy but did seem to improve depressive symptoms when taken in combination with an antidepressant medication. However, Bot and colleagues found that the ω-3 plus antidepressant combination was no better than antidepressant medication alone in decreasing depressive symptoms in patients with depression and comorbid type II diabetes mellitus (Bot et al. 2010).
ω-3 use in treatment of depression is especially appealing when looking for safer alternative treatment to antidepressants in the perinatal period. Hibbeln and Davis suggested that ω-3, in particular DHA, is transferred to the fetus for intrauterine neurodevelopment, and that resultant DHA deficiency in mothers would possibly increase rates of perinatal depression (PD) (Hibbeln and Davis 2009). Nine studies examined ω-3 supplementation effects on PD. Four studies with a total of 430 patients found a decrease in overall rates of PD with ω-3 use, however their designs vary significantly. Two of the studies investigated healthy patients with no psychiatric history (Freeman et al. 2006b; Krauss-Etschmann et al. 2007), another study included only patients with PD (Su et al. 2008), while Mozurkewich studied both healthy subjects and those experiencing “psychological distress” (Lucas et al. 2009). They found significantly decreased rates of depression in response to ω-3 supplementation only in healthy subjects not currently in a major depressive episode (MDE), but no benefit to patients in an MDE. The remaining five studies included a total of 455 patients, and failed to find a benefit for ω-3 supplementation, but only three of these studies included women with diagnosed PD (Su et al. 2008; Freeman et al. 2008; Marangell et al. 2004). Taken as a whole, these studies suggest that this may be a small effect size benefit associated with ω-3 therapy for MDD. This may be due to the heterogeneity of the patients enrolled in those trials.
Bipolar Disorder
Stoll et al. reported that ω-3 supplementation markedly decreased depressive symptoms in bipolar patients (Stoll et al. 1999). Because of the public health need for developing effective treatments for bipolar depression that did not increase the risk for mania or metabolic syndrome, there was tremendous enthusiasm for this work. It led to a series of ω-3 augmentation studies for bipolar disorder (Table 18.2). Three studies found a significant decrease in depression severity, but none showed benefit in mania. Four other studies failed to substantiate these findings demonstrating no efficacy for depressive or manic symptoms with ω-3 supplementation.
Table 18.2
ω-3 efficacy in bipolar disorder
Study | Population | N | Intervention | Duration (weeks) | Primary outcome measure | Results | Comments |
|---|---|---|---|---|---|---|---|
Chiu et al. (2005) | BPAD I, acute mania | 15 | EPA/DHA 6.8 g; PLB | 4 | YMRS | No difference | |
Clayton et al. (2009) | Adolescents; BPAD I & II, Bipolar NOS | 18 | EPA/DHA 1 g | 6 | YMRS, HRDS | Decreased depression | No benefit in mania |
Frangou et al. (2006) | BPAD I & II | 75 | EPA 1 g; EPA 2 g; PLB | 12 | HDRS | Decreased depression | Sig. decrease in primary outcome, but not secondary |
Gracious et al. (2010) | BPAD I & II | 51 | ALA 6.6 g; PLB | 16 | CDRS | No difference | |
Keck et al. (2006) | BPAD I & II, Bipolar NOS | 121 | EPA 6 g; PLB | 16 | IDS-C | No difference | |
Murphy et al. (2012) | BPAD I | 45 | CYT 2 g + ω-3 4 g; ω-3 4 g; PLB | 16 | Retention in study | No difference | Secondary outcomes: MADRS, YMRS, GAF—no difference |
Stoll et al. (1999) | Mania or depression | 44 | EPA/DHA 9.6 g; PLB | 16 | HDRS | Decreased depression | Sig. decrease in primary outcome, but not secondary |
Mood Disorder Meta-analyses
Ten meta-analyses evaluated available randomized controlled trials of ω-3 supplementation in mood disorders (Table 18.3). Two meta-analyses, Bloch and Hannestad, and Rogers et al., included 13 separate RCTs investigating ω-3 therapy for patients with MDD and reported no significant benefit (Rogers et al. 2008; Bloch and Hannestad 2012). A Cochrane Review in perinatal depression by Dennis and Dowswell also found no significant benefit with ω-3 use in perinatal depression (Dennis et al. 2013). Montgomery and Richardson, and Sarris et al. examined RCTs comparing ω-3 supplementation for bipolar depression versus bipolar mania (Montgomery and Richardson 2008; Sarris et al. 2012). They both found overall benefit for ω-3 supplementation in bipolar depression but not in mania. Five other meta-analyses included multiple psychiatric disorders (Appleton et al. 2006; Freeman 2006; Kraguljac et al. 2009; Sublette et al. 2011; Lin and Su 2007). Freeman, Lin et al., and Kraguljac et al. determined there was significant effectiveness of ω-3 in both unipolar and bipolar depression, while Appleton et al. failed to find benefit in psychiatric disorders generally. Sublette et al. included a majority of MDD studies (n = 12) with only one BPAD study, and concluded there was a role for EPA augmentation, but not DHA, in unipolar depression. In general, these meta-analyses suggest: (1) ω-3 monotherapy for mood disorders is not effective for a heterogeneous group of patients with MDD or BPAD and (2) that there may be a role for ω-3 as an augmenting agent for some patients with unipolar and bipolar depression.
Table 18.3
Meta-analyses: ω-3 efficacy in treatment of all-type depressive disorders
Study | N | Intervention | Duration (weeks) | Results |
|---|---|---|---|---|
Appleton et al. (2006) | MDD = 7 Bipolar = 2
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|


