Positive Airway Pressure Treatment for Sleep Apnea
Richard B. Berry
HISTORY OF POSITIVE AIRWAY PRESSURE
Sullivan and coworkers (1) published the original description of nasal continuous positive airway pressure (CPAP) for the treatment of obstructive sleep apnea (OSA) in adults in 1981. Prior to that report, the only effective treatment for moderate to severe OSA was a tracheostomy. Today, positive airway pressure (PAP) is the mainstay of treatment for moderate to severe OSA in adults (2,3,4,5 and 6). Patients with mild OSA (7,8,9,10,11 and 12), the upper-airway resistance syndrome (13), or even heavy snoring (14,15) have also been treated successfully with positive pressure. Since the original description of CPAP, other methods for delivering PAP have been developed, including bilevel PAP (16), autoadjusting positive airway pressure (APAP) (17,18 and 19), and expiratory pressure relief (Cflex). Many types of interfaces for the delivery of positive pressure, including nasal masks (14,20,21), nasal prong interfaces (22), masks covering both nose and mouth (23,24 and 25), and oral interfaces (26), have been developed. However, even after many technological improvements, the overall therapeutic efficacy of PAP is still critically dependent on a knowledgeable physician directing care and an organized and responsive structure of support personnel (27).
MECHANISMS OF ACTION
It is currently believed that PAP maintains upper airway patency primarily by the “pneumatic splint mechanism.” This refers to a simple dilation of the airway by intraluminal pressure that is positive compared with atmospheric pressure. Both computed axial tomography and magnetic resonance imaging have clearly shown that positive pressure increases upper-airway cross-sectional area and volume (28,29 and 30). The major configurational change appears to be an expansion of the airway in the lateral directions. Upper airway muscle activity tends to actually decrease once the upper airway is splinted open (28,31,32), suggesting that the effect of CPAP on upper-airway patency, in general, does not depend on upper-airway muscle activity. A second possible mechanism is an indirect action on the upper airway mediated by an increase in lung volume induced by PAP. Upper-airway size increases as lung volume increases (33). One explanation for this effect is that as lung volume increases, there is a downward traction on the trachea (tracheal tug) and other upper-airway structures (34). By an undefined mechanism, possibly related to stretching or stiffening of upper airway walls, this pull tends to open the airway. A study of one patient with OSA found a decrease in the apnea-hypopnea index (AHI) with negative pressure around the chest and the abdomen (35). However, another study found that an increase in functional residual capacity (FRC) by subatmospheric pressure via a negative pressure body suit did not decrease the frequency of obstructive events (36). A third study found little decrease in upper-airway resistance with the application of negative extrathoracic pressure (37). It appears that most of the effect of CPAP on the upper airway is a direct consequence of the “pneumatic splint.” Of note, one study did find a decrease in obstructive events when only expiratory positive airway pressure (EPAP) was applied (38). Thus, pneumatic splinting, even when confined to expiration, has some benefit.
Positive Pressure Modes—An Overview
CPAP stabilizes the upper airway by maintaining a nearly constant positive pressure in both inspiration and exhalation (Fig. 20-1). In bilevel pressure, there are two independently adjusted pressures (16). A higher pressure is provided during inspiration (inspiratory positive airway pressure [IPAP]) and a lower pressure during exhalation (EPAP). The IPAP-EPAP pressure difference is the amount of pressure support provided to assist inspiration. For example, a bilevel PAP of 15/5 cm H2O provides an IPAP of 15 cm H2O, an EPAP of 5 cm H2O, and a pressure support of 10 cm H2O. Bilevel pressure has two potential advantages over CPAP. First, airway patency can be maintained with a lower pressure during exhalation. A notable number of patients find CPAP uncomfortable, particularly during exhalation, and may find the lower expiratory pressure more tolerable. Second, the IPAP-EPAP difference provides pressure support for inspiration and can augment ventilation. This feature is potentially very useful in patients who hypoventilate during sleep or benefit from pressure support because of lung disease, muscle weakness, or chest wall deformity such as kyphoscoliosis. Although one study failed to find that bilevel PAP increased compliance compared with CPAP (39), individual patients may accept bilevel PAP treatment but not CPAP. There may also be subsets of patients with OSA who tend to prefer bilevel PAP. A study by Resta and coworkers (40) suggested that bilevel PAP may be a better treatment than CPAP in the subsets of patients with OSA who have chronic obstructive pulmonary disease (COPD) or the obesity hypoventilation syndrome (OHS) (38). Of note, although uncommonly used in patients with OSA, bilevel devices can also be used with a backup rate (spontaneous timed [ST] mode). If the patient’s respiratory rate exceeds the backup rate, the bilevel devices function as usual in spontaneous (S) mode. If the patient’s respiratory rate drops below the backup rate, the machine will cycle to IPAP. The duration of IPAP and EPAP are determined as in the S mode. The machines will also cycle from IPAP to EPAP if no exhalation is sensed once the IPAP max time is reached. The bilevel PAP timed (T) mode is very rarely used in sleep apnea but can be used to treat hypoventilation. In this mode, the device delivers IPAP-EPAP cycles only at the set rate. The relative proportion of IPAP-EPAP for the machine breath is set by the clinician. One study suggested that patients with OSA who continue to hypoventilate may benefit from bilevel PAP in the T mode (41). However, this study did not compare the efficacy of the ST and T modes.
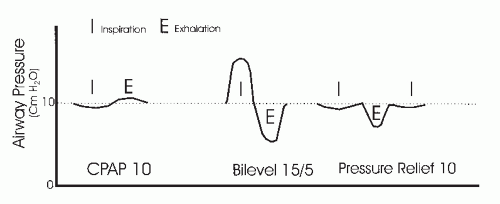 FIGURE 20-1 Positive pressure modes. CPAP, bilevel PAP, and CPAP with pressure relief (Cflex) are illustrated. |
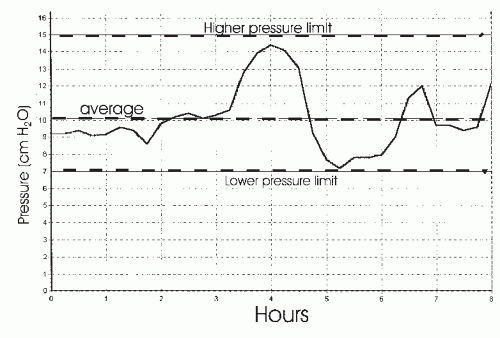
A common feature of both CPAP and bilevel PAP treatment is that a fixed level of pressure(s) is delivered during the entire night. This requires that an “optimal” level of fixed pressure(s) must be sufficient in all body positions and sleep stages. However, it has been recognized since the early days of positive pressure treatment that higher pressure is typically needed in the supine position (42,43 and 44). This has been well documented in systematic comparisons. In addition, higher pressure may be needed during REM sleep in many patients (42). Alternatively, some patients might require substantially less pressure in the lateral sleeping position or with the head elevated (44). Thus, a fixed pressure(s) may deliver higher than needed pressure to some patients during variable portions of the night. In theory, the average nightly pressure would be lower if only the minimum pressure required to maintain upper-airway patency was administered at any given time during sleep. It has been postulated that a lower average overnight pressure would increase adherence in some patients. Attended PAP titration to determine the optimal pressure is also a labor-intense procedure that may not be available in all circumstances. These difficulties associated with fixed PAP treatment and attended PAP titration resulted in the development of autotitrating APAP devices (17,18 and 19,45,46,47,48 and 49). One or more of the following respiratory variables are typically monitored by a given APAP device: airflow magnitude (46), inspiratory airflow flattening (airflow limitation) (48), airway vibration/snoring (47), and airway impedance (49). The algorithms for pressure change vary between different devices. Pressure changes are made gradually to avoid inducing arousal. For example, the presence of snoring could prompt a pressure increase of 0.5 cm H2O over 1 minute. If none of the monitored variables is detected, the devices also begin to slowly lower pressure. Thus, APAP devices deliver the lowest effective pressure required in a given circumstance. The clinician sets the lower and upper pressure limits, and the device will deliver pressure as needed between these two
limits (Fig. 20-2). High mask or mouth leak can simulate physiologic events and result in errors in APAP titration. One report of a large experience with the APAP devices estimated that leak exceeded 0.4 L/s (24 L/min) in about 10% of supervised nights and 15% of unsupervised nights (17). The devices also cannot differentiate between central and obstructive apnea. In many published studies of APAP, patients with central apnea or congestive heart failure (CHF) (increased incidence of Cheyne-Stokes breathing [CSB]—central apnea) were excluded (18). The use of APAP devices as an alternative to traditional PAP titration will be discussed in the titration section. The evidence that APAP can increase adherence to PAP will be reviewed in the section on PAP adherence.
limits (Fig. 20-2). High mask or mouth leak can simulate physiologic events and result in errors in APAP titration. One report of a large experience with the APAP devices estimated that leak exceeded 0.4 L/s (24 L/min) in about 10% of supervised nights and 15% of unsupervised nights (17). The devices also cannot differentiate between central and obstructive apnea. In many published studies of APAP, patients with central apnea or congestive heart failure (CHF) (increased incidence of Cheyne-Stokes breathing [CSB]—central apnea) were excluded (18). The use of APAP devices as an alternative to traditional PAP titration will be discussed in the titration section. The evidence that APAP can increase adherence to PAP will be reviewed in the section on PAP adherence.
The newest method of PAP delivery is a variant of CPAP, expiratory pressure relief (Cflex), which allows the airway pressure to fall during expiration in proportion to expiratory airflow. The airway pressure returns to the prescribed CPAP level at end exhalation. Patients may select one of three levels of pressure relief (e.g., different relationships between expiratory flow and the amount of pressure decrease). This treatment may be useful in pressure-intolerant patients. To date, no information has been published regarding the impact on adherence to treatment. As will be discussed in later sections, pressure intolerance is not the most common PAP side effect.
INTERFACES FOR POSITIVE AIRWAY PRESSURE
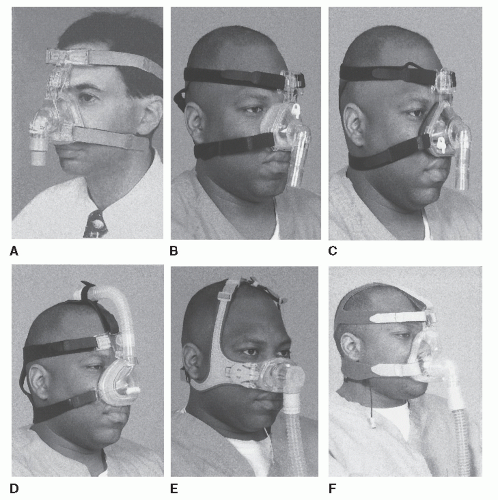
Developing an optimal interface between the airflow generator or blower and patient has been the source of tremendous challenge. The PAP industry has recognized this problem, and currently, a large variety of alternatives are available (Figs. 18-3 and 18-4). However, finding an adequate interface remains a significant problem. Mask discomfort (pain from pressure on the nose, cheeks, or upper lip), air leak (noise, eye irritation), and shift in mask position with body position changes may all impair sleep quality. In the original description of CPAP, small cannulalike tubes were placed in the nostrils and held in place with polymer (1). Later, the idea of a nasal mask was introduced (14,20,21). In many patients, mouth leaks are not problematic when using a nasal interface because the positive pressure in the nasopharynx moves the soft palate forward against the tongue, sealing off the oral cavity. Otherwise, the patient must maintain or be assisted to maintain a closed mouth (chinstrap) or utilize an oronasal mask, which simultaneously applies pressure to the nasal and oral airways.
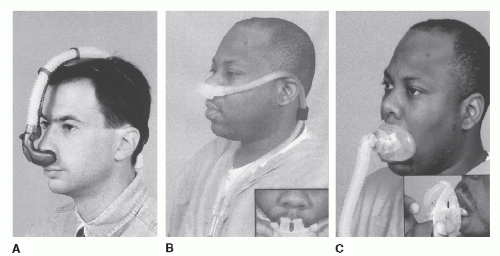
Typically, a given type of mask is produced in a series of sizes (small, medium, large), but these rarely provide good fits for all facial structures. Material used for the mask are either compliant and bulge out from pressure against the face (Fig. 20-3A,B,D, and F) or are composed of a soft gel material (Fig. 20-3C). A particular challenge is obtaining a good seal over the bridge of the nose. Leaks of air in this area typically irritate the eyes (50). Patients without upper teeth may have limited structural support for the upper lip area. In some of these patients, wearing dentures at night may help. To resolve these mask problems, several versions of interfaces have been developed to provide a seal in the nares, such as nasal pillows (Fig. 20-4A) or nasal prongs (Fig. 20-4B) (22). Two other advantages for this type of device are that patients can read with the interface in place and typically feel less claustrophobic. Another method of handling similar problems is the design of “mini masks” that fit only over the tip/lower portion of the nose (Fig. 20-3E).
If patients have significant nasal congestion or obstruction, they may find breathing through a nasal mask with the mouth closed very uncomfortable. For this reason, oronasal or full-face masks covering both the nose and mouth (Fig. 20-3F) have been developed (23,24 and 25). These masks incorporate valves that allow inspiration of room air should the pressure source fail. They also have head straps that allow quick removal as a precaution against vomiting or other events requiring rapid mask removal. One problem with these full-face masks is that they are required to form a good seal over a larger area of face. Thus, obtaining a good mask fit in all patients has been
a problem. The full-face mask can be particularly useful in patients with mouth leak and also tend to prevent the drying of the upper airway. In some patients initially requiring a full-face mask, intensive medical or surgical treatment of the nasal airway may allow eventual conversion to a nasal mask. Recently oral interfaces (Fig. 20-4C) have also been developed for PAP (26). The nose is completely bypassed but aggressive humidification is required to prevent dryness. Nasal leak can be a problem with oral interfaces just as a mouth leak can complicate treatment with a nasal mask.
a problem. The full-face mask can be particularly useful in patients with mouth leak and also tend to prevent the drying of the upper airway. In some patients initially requiring a full-face mask, intensive medical or surgical treatment of the nasal airway may allow eventual conversion to a nasal mask. Recently oral interfaces (Fig. 20-4C) have also been developed for PAP (26). The nose is completely bypassed but aggressive humidification is required to prevent dryness. Nasal leak can be a problem with oral interfaces just as a mouth leak can complicate treatment with a nasal mask.
Because all modern CPAP equipment uses a single hose between blower and mask, some mechanism to wash out the exhaled CO2 is needed. This is commonly performed by placing a small orifice or slit(s) in the nasal or full-face mask. Of note, the amount of leak (intentional leak) can increase considerably with increases in pressure. Although the possibility of rebreathing has been a concern if the mask is operating at lower pressures (less intentional leak), this does not appear to be a significant clinical problem. Most patients have some degree of unintentional leak, and the orifices are large enough to provide adequate washout at low pressures. On the other hand, the amount of intentional leak at high pressures can be up to 40 L/min with some masks.
ANCILLARY POSITIVE AIRWAY PRESSURE OPTIONS
The RAMP option is available on most CPAP and bilevel devices (4). This allows the patient to select a “ramp time” during which the pressure slowly increases from a low initial value (typically 4 cm H2O) to the prescription pressure. This provides a window of time during which pressure lower than the target pressure is applied and offers the patient an opportunity to fall asleep. The pressure from which a ramp may start to climb may be adjusted on some types of devices. This is a useful option because some patients prefer an initial pressure higher than 4 cm H2O. Although the RAMP option makes sense, it has never been shown to increase adherence. Many PAP devices also offer mask-off alarms to alert the patient that the mask has been pulled off. The utility of this feature has never been systematically studied. Altitude compensation and adjustment for variable voltage sources are also available on many PAP units. Altitude compensation may be automatic or user selectable. This is a valuable feature because the same blower speed at higher altitudes delivers less pressure because of the decreased air density (51). If the patient develops a complaint of the pressure “feeling different,” the altitude adjustment should be checked to make sure that it was not inadvertently changed.
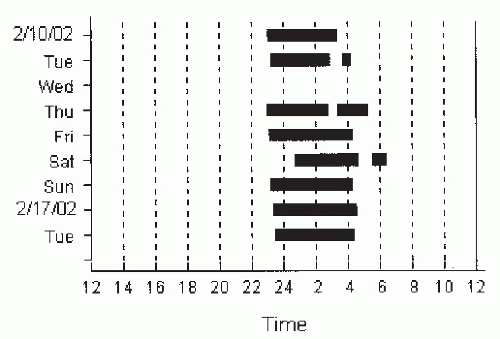
Currently, all but the lowest-priced PAP devices offer some method of objectively monitoring patient use (adherence) (52,53). As will be discussed later, the capacity to objectively monitor PAP use is essential for optimal clinical care. The most basic method is a simple runtime meter (recording time the device is turned on). The average hours per night the machine is turned on can be calculated by the change in the meter values divided by the number of elapsed days. More advanced machines can monitor actual use (“time at pressure” or time with varying blower speed), and computer chip technology has allowed the recording of an electronic diary of the exact dates and times the machine was used (Fig. 20-5). This feature allows the physician to detect skipped days or early removal of the PAP device (the 4 AM trip to the bathroom). Some devices will also record estimates of persistent events or leak information. This stored machine information can be assessed through memory cards, modems, or direct transfer of information from the unit to a computer.
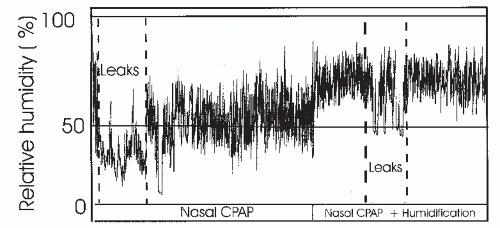
The use of humidification has been another frequently useful development. Initially, cold passover humidification (air simply blown across a water reservoir) was available. However, more moisture can be delivered with heated humidification and, currently, stand-alone heated humidifiers or models incorporated into the PAP device (integrated humidifier) are available. Studies have shown that nasal CPAP results in some drop in the relative humidity of air in the upper airway. Mouth leak causes a more dramatic drop in mask humidity via a unidirectional escape of water vapor out of the mouth (54,55) (Fig. 20-6). In fact, the symptom of mouth dryness usually implies some degree of mouth leak. Dryness of the nasal mucosa results in a large increase in nasal resistance (55). This tends to cause more mouth opening and greater leak. In order to prevent this cycle, adequate humidification of air is required. One study found that heated but not cold humidity prevented changes in nasal resistance secondary to large mouth
leak (55). Humidification by preventing dryness may also reduce the nasal congestion some patients experience when using CPAP. In one study, humidification increased average nightly use and patient satisfaction with CPAP treatment (heated > cool > none) (56). Those patients who benefited the most were those with symptoms of nasal congestion or dryness at baseline. This study could be criticized for the fact that the subjects were not blinded to the fact that no humidity was used in the control arm. Medical devices can have an appreciable placebo effect. Another study found that prophylactic use of humidity to all comers did not improve satisfaction with the initial attended PAP titration in the sleep laboratory (57). Thus, there is conflicting evidence on whether or not humidity should be used on every patient. It seems prudent to target humidification toward patients most likely to benefit (nasal congestion dryness and mouth leak). If there is a problem with condensation in the tubing, a tube insulator may be of some benefit. Certainly, care of humidifiers (cleaning and filling with water) requires some extra patient effort. Adherence data on the use of humidifiers is not available.
leak (55). Humidification by preventing dryness may also reduce the nasal congestion some patients experience when using CPAP. In one study, humidification increased average nightly use and patient satisfaction with CPAP treatment (heated > cool > none) (56). Those patients who benefited the most were those with symptoms of nasal congestion or dryness at baseline. This study could be criticized for the fact that the subjects were not blinded to the fact that no humidity was used in the control arm. Medical devices can have an appreciable placebo effect. Another study found that prophylactic use of humidity to all comers did not improve satisfaction with the initial attended PAP titration in the sleep laboratory (57). Thus, there is conflicting evidence on whether or not humidity should be used on every patient. It seems prudent to target humidification toward patients most likely to benefit (nasal congestion dryness and mouth leak). If there is a problem with condensation in the tubing, a tube insulator may be of some benefit. Certainly, care of humidifiers (cleaning and filling with water) requires some extra patient effort. Adherence data on the use of humidifiers is not available.
PATIENT SELECTION FOR POSITIVE AIRWAY PRESSURE
There are three major reasons to treat OSA (58): (a) To decrease symptoms including excessive daytime sleepiness (EDS) (5) and associated morbidity such as an increased risk of auto accidents (59,60), (b) to prevent long-term cardiovascular and cerebrovascular consequences of OSA (61,62,63,64,65,66 and 67), and (c) to improve bed partner sleep (alleviate socially unacceptable snoring) (68). Several controlled studies have demonstrated that PAP is effective in decreasing subjective and objective sleepiness and improving the quality of life in symptomatic patients with moderate to severe disease (5,6). In general, although PAP can be effective in some patients with mild OSA (or those with isolated snoring), this group tends to be much less adherent to PAP treatment (7,8,9,10,11 and 12). Patient preference should also be considered. Some patients with milder disease may not want upper-airway surgery or an oral appliance. In these cases, choices are limited to conservative treatment (weight loss, etc.) or positive pressure.
The indication for PAP treatment of asymptomatic patients is more controversial. In one 6-week study, there was no benefit with regard to sleepiness, quality of life, or cognitive test performance in moderate to severe cases of OSA (AHI of >30 per hour) when patients were not sleepy (69). In this study, there was also no improvement in blood pressure, a finding that conflicts with the results of some other studies (66,67). In addition, the severity of arterial oxygen desaturation was not specified. The results may then not be applicable to all patient groups. Social reasons for effective treatment can be very compelling for some patients. Some increased risk of developing cerebrovascular or cardiovascular disease exists even at low AHI levels (64,65). However, no prospective controlled study has yet demonstrated that CPAP treatment will decrease the risk of long-term consequences. The risk of long-term consequences of untreated OSA in a given patient cannot be estimated with certainty. However, the presence of more severe arterial oxygen desaturation or comorbid conditions (coronary artery disease, CHF, prior cerebrovascular accidents [CVAs]) may well increase risk.
Medicare guidelines for the reimbursement of PAP treatment have recently been revised (70). For patients with an AHI of 5 to 14 events per hour, symptoms or signs of significant impairment are required. The qualifying impairments include daytime sleepiness, insomnia, hypertension, prior CVA, or a mood disorder. For patients with an AHI of 15 events per hour or more, no associated findings are required. Of note, the Medicare definition of hypopnea is stringent. A hypopnea is defined as a ≥30% reduction in flow followed by a ≥4% desaturation. Arousals are not considered in this definition of hypopnea. No associated desaturation is required for an apnea.
In summary, one can argue that all patients with moderate to severe OSA, with or without symptoms, should be offered PAP treatment. Most would also offer PAP to symptomatic patients with mild OSA as an alternative to other treatments. For patients with moderate to severe
OSA without symptoms, the most compelling reasons for treatment would be social acceptance and prevention of long-term consequences. Scientific evidence (see later section) is accumulating that asymptomatic patients with significant cardiovascular comorbidity probably have the most to gain from PAP treatment.
OSA without symptoms, the most compelling reasons for treatment would be social acceptance and prevention of long-term consequences. Scientific evidence (see later section) is accumulating that asymptomatic patients with significant cardiovascular comorbidity probably have the most to gain from PAP treatment.
TABLE 20-1 ESSENTIALS OF POSITIVE PRESSURE TITRATION | |||||
|---|---|---|---|---|---|
|
POSITIVE PRESSURE TITRATION
The pressure used for PAP treatment is usually determined by a positive pressure titration (Table 20-1). Pressure is incrementally increased until an optimal pressure is reached. Standards for positive pressure titration have been published by a number of organizations (71,72). Attended polysomnography (PSG) including electroencephalograph monitoring has been the standard of care for titration. This allows a determination of sleep stage and body position (42,43 and 44). The sleep technologist is available for adjustments in mask seal and to minimize discomfort and patient complaints. Episodes of significant arterial oxygen desaturation that sometimes occur even when airflow is adequate (commonly during REM sleep) (73) or significant arrhythmias can be recognized and addressed. PAP titration is a labor-intensive process, and usually a technologist can titrate only one or two patients at a time.
Although airflow can be monitored with a thermistor or nasal pressure cannula under the mask, most sleep centers today monitor a flow signal generated by the positive pressure device (sometimes called machine flow, CPAP flow, Vest, or Cflow). This signal provides accurate information about the magnitude of flow and airflow limitation (flattened airflow profile). Some airflow signals may also provide snoring information, depending on the filtering and frequency response of the measuring device in the machine. An estimated leak signal as well as machine pressure are also available (74). The use of the leak signal will be discussed in the following section.
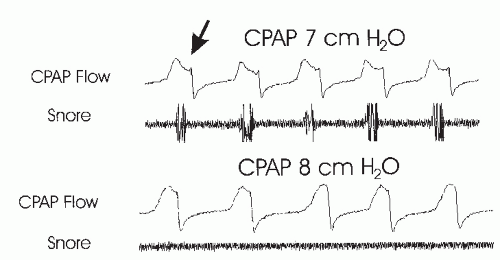
It has been recognized that both snoring and airflow limitation usually imply persistent high upper-airway resistance (75,76) and are clues that a higher pressure may be needed. In Figure 20-7, a small increase in CPAP eliminates snoring and evidence of airflow limitation in the flow signal. The end point (goal) of titration varies somewhat but ideally includes elimination (or near elimination) of obstructive apnea, hypopnea, snoring, arterial oxygen desaturation, and respiratory effort-related arousals (RERAs), which are those events associated with arousals secondary to increased respiratory effort that do not meet criteria for obstructive hypopneas (77). As esophageal pressure is not typically measured, RERAs are usually inferred from the evidence of airflow limitation or snoring preceding an arousal. Some centers also try to eliminate all evidence of airflow limitation (77). Central apneas and mild airflow limitation may be acceptable as long as there is no associated sleep disturbance or significant oxygen desaturation. The goal of most centers is to have an AHI of <5 events per hour in all body positions and sleep stages. The highest pressures are usually needed during supine REM sleep. Unfortunately, supine REM sleep may not be recorded in every patient.
Bilevel pressure titration protocols vary, but two methods are popular (16). One can increase IPAP-EPAP until obstructive apneas are abolished. Further increases in IPAP are administered until hypopnea/snoring is
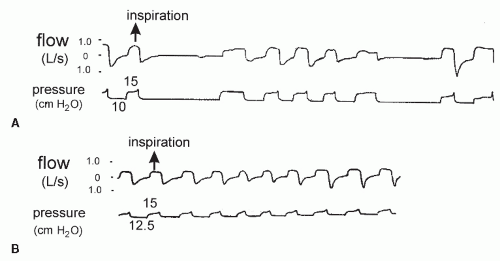
eliminated. Alternatively, one could start with 6/4 to 8/4 and then sequentially increase IPAP and EPAP as needed. In general, apnea requires an increase in EPAP (Fig. 20-8). A common error is to attempt to stabilize the airway with EPAP that is too low or an IPAP-EPAP differential that is too high.
eliminated. Alternatively, one could start with 6/4 to 8/4 and then sequentially increase IPAP and EPAP as needed. In general, apnea requires an increase in EPAP (Fig. 20-8). A common error is to attempt to stabilize the airway with EPAP that is too low or an IPAP-EPAP differential that is too high.
Initially, PAP titrations were performed during an entire night that followed a previous diagnostic study. This allowed an entire night for diagnosis and estimation of severity. Frequently, the patient could be educated by a physician about the nature of his problem, treatment alternatives, and the nature of PAP treatment before the PAP titration. However, because of limited resources and to expedite treatment, split- or partial-night studies were introduced (78,79,80,81 and 82). The first portion of the night is for diagnosis, and the second part is for the PAP titration. Obviously, this approach allows less time for each goal, diagnosis, and PAP titration. Several studies have assessed the ability of a splitnight approach to determine an effective level of PAP, and guidelines for patient selection have been published by the American Academy of Sleep Medicine (71). In general, around 60% to 80% of patients can be handled adequately by the split-night approach. Criteria for patients to have a split study are listed in Table 20-2. The rationale for these criteria is that severe patients usually do not require either REM sleep or the supine position to manifest the severity of their problem. The severity of mild to moderate cases may be underestimated when adequate amounts of supine or REM sleep are not included in the diagnostic portion of the study. In addition, there appears to be more uncertainty in the optimal level of PAP in milder cases studied for only part of the night. Certainly, enough time must be left for an adequate PAP titration once the diagnosis of OSA has been made. The split-study approach requires education, mask fitting, and optimally, some brief period of PAP adaptation before lights out. If a patient does not meet criteria during the first part of the night, the entire study is performed as a diagnostic one. Autotitrating devices offer an alternative to traditional PAP titration (18,19,83,84,85,86,87 and 88). Information stored in the device memory can be analyzed, and a pressure can be chosen for fixed CPAP. A common method is to choose the 90th or 95th percentile pressure (pressure exceeded only 10% or 5% of the time, respectively) as the prescription pressure. This assumes periods of high leak have been eliminated from analysis. The devices could be used in an attended setting as a technologist extender. Interventions for mask leak, arterial oxygen desaturation, or arrhythmia would then be possible. In this setting, the ability of the devices to provide an adequate titration (low residual AHI) has been well documented (18,19,84,85). Of note, most studies excluded patients with CHF or significant lung disease. The ability of APAP devices to perform in the unattended setting in CPAP naive patients (18,86,87 and 88) has been less well documented. Nevertheless, adequate titration can probably be obtained in many patients. Simply looking at a data summary can be misleading. Most devices allow the ability to look at single-night data in detail (pressure, leak, residual events vs. time) (Fig. 20-9). Periods of high leak and the frequently associated increase in pressure can be appreciated. High leak can result in many devices promptly increasing pressure until the upper pressure limit is reached. In Figure 20-9, a single-night tracing on APAP shows high leak (>0.4 L/s). Patients with suboptimal or inconclusive APAP titrations should have an attended lab PAP titration.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree