, Damien Gervasoni1 and Catherine Vogt2
(1)
Lyon Neuroscience Research Centre CNRS UMR 5292–INSERM U 1028, Université Claude Bernard Lyon 1, Lyon, France
(2)
Université Claude Bernard Lyon 1, Lyon, France
At the end of a whole animal experiment, postmortem examination is necessary to validate the experimental data. Steps include euthanasia, autopsy and removal of the brain, treatments specific to organ fixation, sectioning, and staining.
7.1 Different Types of Euthanasia Accepted by Legislation (Directive 2010/63)
The term “euthanasia” is derived from the Greek eu (good) and thanatos (death) and literally means a “good death,” ethically speaking.
Accordingly, methods of humane euthanasia must comply with a number of principles. While being compatible with the experiment, the method chosen must also bring about death without pain or signs of panic or stress. Loss of consciousness must be quick or instantaneous. The technique must be reliable and reproducible but also proven safe for the animal handler. Euthanasia must be performed in a place separate from premises where the animals are normally housed.
The method of euthanasia most commonly used in neuroscience is anesthetic overdose. This consists of administering several times the dose of an anesthetic, such that a rapid loss of consciousness is induced and death by cardiopulmonary arrest is provoked.
Cervical dislocation consists of provoking injury of the cervical spinal cord and rupture of the blood vessels supplying the brain. Death is rapid and without pharmacological interference (from anesthetics, analgesics, etc.). However, the technique requires experience and is usually reserved for mice and rats smaller than 200 g body weight.
Decapitation meets similar criteria as cervical dislocation. The procedure consists of cutting off the head of the animal at its base, using the appropriate instrument: a very sharp and strong knife blade or guillotine, which should be thoroughly cleaned after each use, so as not to stress subsequent animals by the smell of blood. The gesture of execution must be confident and without hesitation.
It is always preferable to carry out the above two procedures after the animals have already been anesthetized.
Euthanasia with carbon dioxide is the method of choice for rodents, especially when carrying out the procedure on numerous experimental groups. Loss of consciousness is rapid and death occurs within 2–3 min.
Exsanguination is used only under deep anesthesia. This method allows for the collection of a large volume of blood sometimes necessary for certain experimental measurements and at the same time permits preparation of the animal for organ fixation by perfusion.
Note: After euthanasia, it is imperative to verify the death of the animal.
7.2 Perfusion
Fixation of the brain by perfusion is usually performed under deep anesthesia of the animal while the heart is still beating. The procedure requires the use of the appropriate instruments (Fig. 7.1) to access the thoracic cavity and to put the perfusion catheters in place.
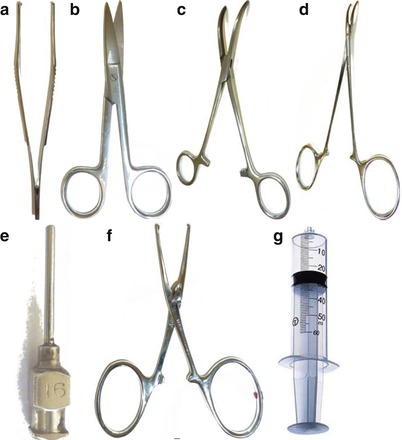

Fig. 7.1
Instruments used for the perfusion and removal of the brain. (a) Toothed tissue dissecting forceps. (b) Mayo operating scissors. (c) Curved hemostatic forceps. (d) Needle holder. (e) Round-tipped (blunt) syringe needle. (f) Straight hemostatic forceps. (g) 50 ml syringe
Once deep anesthesia has been induced, the thorax is opened. Using a hemostatic forceps (Fig. 7.1c), the xiphoid process (the inferior part of the sternum) is gripped through the skin. By applying a slight upward traction, the costal arch (or costal margin) can be visualized under the skin. An incision with scissors is made obliquely through the skin, muscle, and the ribcage on both sides of the sternum (Fig. 7.2a). The diaphragm is freed at its lateral insertions in order to retract the anterior wall of the thorax towards the head of the animal (Fig. 7.2b).
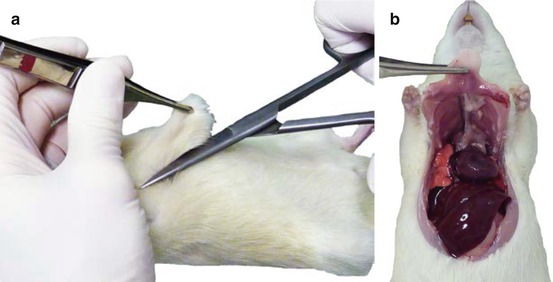

Fig. 7.2
(a) Gripping the sternum with forceps before the incision with operating scissors. (b) Retraction of the rib cage towards the head of the animal after lateral incisions of the diaphragm
This surgical approach gives wide access to all of the thoracic organs, especially the heart. The heart is retracted to the left in order to see the thoracic aorta, which is clamped entirely without prior dissection. In this way, the posterior part of the animal is isolated from a vascular point of view, leaving the volume of the intracardiac perfusion to distribute only within the anterior part. The perfusion needle (see Fig. 7.1e) is inserted into the left ventricular muscle and slipped inside the ventricular cavity up to the start of the aorta. In order not to damage the aorta itself, a blunt round-tipped needle should be used. Once the needle is in place, it is clamped together with the heart with the aid of the second pair of hemostatic forceps (Fig. 7.1f), in order to maintain the needle in place within the heart during the perfusion. The right atrium is partially sectioned with scissors to allow for the venous outflow of the perfusion liquid. The perfusion is initiated with 50 ml of physiological saline to eliminate a maximum amount of blood from the arteriovenous cerebral circulatory system.
Perfusion then continues with 50 ml of a 4 % paraformaldehyde solution to prefix the organ. All of the following procedures should be performed under a vertical laminar flow hood to avoid the risk of exposure to formaldehyde vapors.
With whatever liquid used, the perfusion can be performed manually if it is done slowly enough to minimize intravascular back pressure. However, it is preferable to use a peristaltic pump adjusted to a fixed maximum flow rate of 20 ml/min. A successful perfusion of paraformaldehyde is evident when it reaches the muscles of the forelimbs. At this point, spasmodic movements of the muscle are induced by the conformational change of muscle protein in reaction to the fixative. Formaldehyde is an electrophilic substance that binds covalently upon contact with proteins (rich in nucleophilic sites) causing their denaturation. At the end of the perfusion, the body of the animal becomes rigid and can then be decapitated using a guillotine.
7.3 Removal of the Brain
To ensure an optimum quality of fixation, the brain is removed before the rest of the body is examined. Figure 7.3 illustrates the equipment required. Once the head has been removed using a guillotine, the scalp is incised medially in order to laterally retract the skin and muscle overlying the skull surface. Bone rongeurs are used to remove pieces of the bone plates, starting at the occipital foramen at the base of the skull.
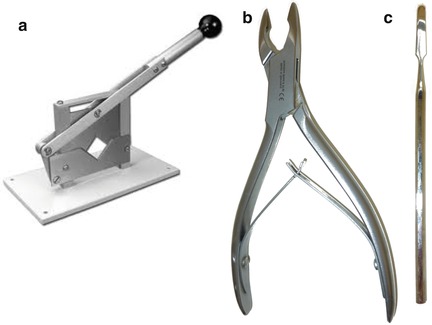

Fig. 7.3
Instruments used for removing the brain. (a) Small animal guillotine. (b) Bone rongeurs. (c) Spatula
Once the cerebellum has been exposed, the extremity of one of the jaws of the rongeurs is carefully inserted between the dura mater and the brain, taking care not to damage the surface of the latter. The various plates of cranial bone are removed one by one by carefully lifting upward from the inside out. When the overlying dura and bone are completely disengaged from the brain, a spatula (see Fig. 7.3j) is used to detach and lift the brain off of the floor of the cranial vault. The brain is then immersed in a fixation solution of 4 % paraformaldehyde at 4° C for 2 h, followed by a 36 h immersion in 30 % sucrose before carrying out the cryoprotection procedure.
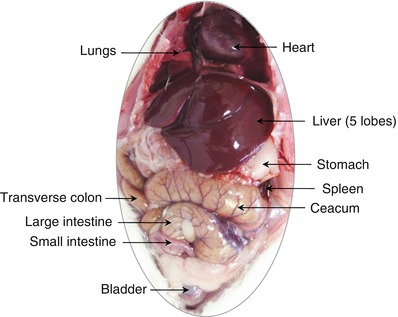
7.4 Autopsy and Examination of the Organs in the Large Body Cavities
At the end of the perfusion, the large (thoracic and abdominal) cavities of the body should be opened to make a rapid assessment of the size, shape, superficial aspect, and color of the various internal organs, in order to eventually reveal any abnormalities in these features (Fig. 7.4) (Piper et al. 2011). The severity of certain observations can serve as a preliminary signal of disease, malformation, or toxicity to the researcher and the attendant veterinarian. If the lesions are associated with a disease state, a diagnosis may be possible through complementary examinations by a diligent practitioner. Depending on the circumstances, it may also be informative to the animal supplier or breeder of a problem or anomaly.