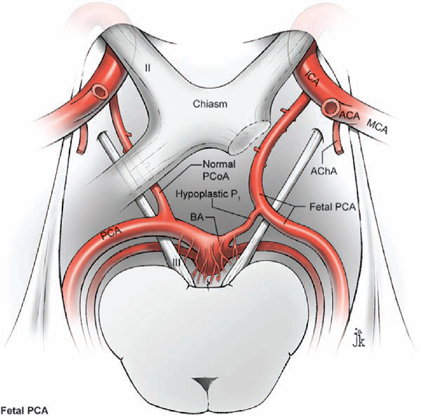
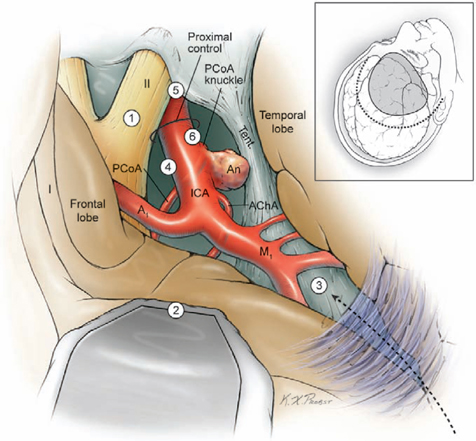
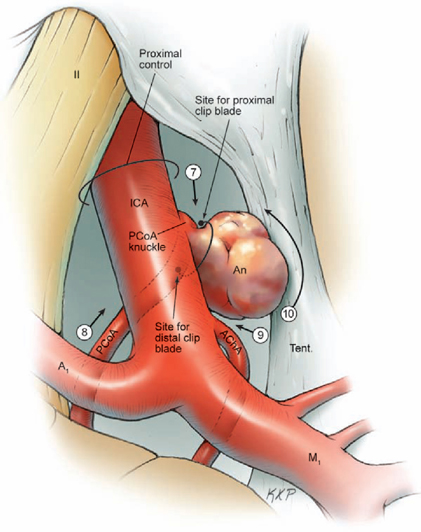
14 Posterior Communicating Artery Aneurysms The posterior communicating artery (PCoA) bisects the supraclinoid internal carotid artery (ICA) into an ophthalmic segment from the distal dural ring to the PCoA, and a communicating segment from the PCoA to ICA bifurcation. The PCoA arises from the posterior carotid wall, courses posteriorly and medially along the superior surface of the oculomotor nerve, and intersects the posterior cerebral artery (PCA) to mark the end of the P1 segment and the beginning of the P2 segment (Fig. 14.1). Approximately eight anterior thalamoperforating arteries originate from the superior surface of the PCoA along its course and ascend to the hypothalamus, anterior thalamus, internal capsule, tuber cinereum, floor of third ventricle, posterior perforated substance, optic chiasm and tract, and pituitary stalk. The anterior choroidal artery (AChA) is the most important branch associated with PCoA aneurysms, lying adjacent to the distal neck. The AChA arises from the posterior wall of the communicating segment, but can also arise from the PCoA, the ICA bifurcation, and the proximal M1 segment. The AChA is usually a single artery, but it can originate as two arteries. Its initial cisternal segment runs posteromedially behind the ICA, following the contour of the medial uncus through the crural cistern and supplying the optic tract, lateral thalamus, and internal capsule (genu and posterior limb). The AChA can assume PCA territories and supply the medial temporal and occipital lobes. The cisternal segment ends at the plexal point where the AChA pierces the telae choroidea of the temporal horn of the lateral ventricle. The plexal segment runs in the temporal horn to the atrium and has diminishing surgical significance. The territory of the cisternal segment makes the AChA so critical, and its compromise causes deficits out of proportion to its small size; these deficits include hemiplegia, hemianesthesia, and hemianopsia. The fetal PCA is an important variant (Fig. 14.2). During embryogenesis, the PCoA initially supplies the occipital lobe, but the P1 segment enlarges to annex this territory and the PCoA shrinks to produce the classic circle of Willis anatomy. The transformation fails to occur in as many as 20% of patients, and the P1 segment remains hypoplastic or atretic. The fetal PCA must be recognized and preserved when clipping PCoA aneurysms because its compromise can result in occipital lobe infarction. The PCoA infundibulum has a “funnel-shaped” PCoA that is dilated at its origin and tapers triangularly, with an artery of normal caliber emerging from the tip rather than from the base, as with true aneurysms (Fig. 14.3). Seen in as many as 10% of patients, this anatomic variant has no proven risk of rupture and is not treated unless it enlarges. Intraoperatively, an infundibulum with a thinned or protuberant posterior wall can be clipped to pinch off this portion of the infundibulum while preserving PCoA flow. The course of the oculomotor nerve parallels the PCoA, and aneurysms projecting posteriorly and inferiorly will impact the nerve, accounting for the third nerve palsies that many patients present with (pupil dilatation and eye deviation laterally and inferiorly, or the “down and out” eye). The nerve originates from the interpeduncular fossa of the mid-brain, courses between the P1 PCA and superior cerebellar artery (SCA), attaches to the membrane of Lilie quist, and climbs to the oculomotor triangle, a dural space bordered by the tentorial edge laterally, the petrous apex posteriorly, and the interclinoidal line medially (the line between the anterior and posterior clinoid processes). The nerve enters its dural sleeve at the triangle’s anterior apex to exit the subarachnoid space and travel in the roof of the cavernous sinus, just below the anterior clinoid process. The optic nerve overlies the supraclinoid ICA at the point of proximal control. A small space is needed for the upper blade of a temporary clip between the inferolateral aspect of the optic nerve and the superomedial aspect of the ICA. The falciform ligament is a dural fold that runs from the medial side of the anterior clinoid process (ACP) to the roof of the optic canal and tuberculum sella. A small incision in the ligament parallel to the lateral edge of the optic nerve expands the space for a temporary clip. The lower blade of a temporary clip fits into the space between the inferolateral aspect of the ICA and the parasellar dura. A large ACP can obscure this space, and its removal helps in visualizing the ICA and facilitating temporary clip application. Fig. 14.1 Microsurgical anatomy of the posterior communicating artery (PCoA), as seen in superior (A) and right lateral (B) overviews. The PCoA bisects the supraclinoid internal carotid artery (ICA) into an ophthalmic segment and a communicating segment, and gives rise to the anterior thalamoperforators. ACA, anterior cerebral artery; AChA, anterior choroidal artery; BA, basilar artery; MCA, middle cerebral artery; OphA, ophthalmic artery; PCA, posterior cerebral artery; SCA, superior cerebellar artery; Tent., tentorium. Fig. 14.2 Microsurgical anatomy of the fetal posterior cerebral artery (superior overview). Fig. 14.3 Microsurgical anatomy of an infundibular origin of the posterior communicating artery (superior overview). Fig. 14.4 Dissection strategy for PCoA aneurysms. Step 1, identification of the optic nerve; step 2, placement of the frontal retractor; step 3, splitting the proximal sylvian fissure; step 4, opening the opticcarotid triangle; step 5, proximal control; step 6, exposing the proximal ICA inferiorly. An, aneurysm. The prevailing surgical attitude toward PCoA aneurysms can be summarized as: “It’s just a P-comm.” Although these common aneurysms require minimal microdissection and have simple anatomy, they should be taken seriously. Their fragile domes have numerous points of adhesion (oculomotor nerve, tentorium, and temporal lobe); the ACP can obscure the PCoA’s origin and the proximal neck; and AChA complications can be devastating. Microdissection begins by identifying the optic nerve through the arachnoid of the chiasmatic cistern (Fig. 14.4, step 1). Even in patients with significant subarachnoid hemorrhage, the optic nerve is recognizable where dura of the anterior cranial fossa floor converges with the cisternal arachnoid. Rotate the table to orient the axis of the ipsilateral optic nerve vertically. A frontal lobe retractor is positioned with the blade’s tip lateral to the olfactory tract as it merges with the optic nerve (Fig. 14.4, step 2). The chiasmatic cistern is opened with a No. 11 blade and the incision is extended with microscissors along the superior surface of the optic nerve and anteromedially to the interoptic triangle. Posterolateral incisions open the carotid and sylvian cisterns. A wide sylvian fissure split is not needed with PCoA aneurysms. Opening the proximal or sphenoidal segment of the sylvian fissure (Fig. 14.4, step 3) separates the frontal and temporal lobes enough to visualize ICA’s communicating segment and proximal M1 segment. Without some sylvian fissure split, the arachnoid of the sylvian cistern overlies this spot. The sylvian arachnoid is lifted and cut with the tip of a No. 11 blade. The sylvian veins emerging from the cistern provide a point of incision into these layers. Blood in the sylvian cistern helps separate the arachnoid from underlying arteries. This sylvian dissection progresses medially to join the earlier dissection of the carotid cistern. This limited dissection of the proximal sylvian fissure and a frontal lobe retractor on the medial orbital gyrus brings the entire supraclinoid ICA into view. This retraction places no traction on the aneurysm. The temporal lobe forms a steep posterior wall in the surgical corridor, and any temptation to retract the temporal lobe should be resisted because PCoA aneurysms projecting laterally above the tentorium often adhere to the uncus and can rupture with such a maneuver. The superior ICA surface is traced anteriorly (Fig. 14.4, step 4) to the apex of the optic-carotid triangle for proximal control (Fig. 14.4, step 5), and if necessary, the falciform ligament is incised to widen the interval between the ICA and the optic nerve. The dissection proceeds laterally around the ICA to its inferior surface (Fig. 14.4, step 6), where there is a space for the lateral blade of a temporary clip proximal to the PCoA origin.
 Microsurgical Anatomy
Microsurgical Anatomy

 Aneurysm Dissection
Aneurysm Dissection
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree