27 | Postlaminectomy Kyphosis |
 | Case Presentation |
History and Physical Examination
A 14-year-boy presented with a complaint of deformity in the form of flexed positioning and pain in his neck. Two years prior to this presentation, the patient had presented with a stiff neck and weakness of his left upper limb. The radiological examination at that time had shown a mass compressing his spinal cord posteriorly at the level of C3-5 (Fig. 27–1). The patient underwent a C3-5 laminectomy with excision of an extradural mass (Fig. 27–2). At pathological analysis, the mass was confirmed to be a Ewing sarcoma tumor. Postoperatively, he was taken to the pediatric intensive care unit where he was observed and placed in a Miami J collar (Jerome Medical, Moorestown, NJ). Later, he was treated with physical therapy and occupational therapy. The patient was discharged home with an intact neurological examination except for left biceps weakness and numbness in the left upper arm and shoulder. The patient’s postsurgical course was uneventful. During the course of the follow-up he also received chemotherapy and radiation therapy. At the time of his initial evaluation in our spine center, the patient was noted to have a kyphotic cervical spine. A computed tomographic (CT) scan of the cervical spine showed a kyphotic deformity of 39 degrees centered at the C4 level. This had progressed from 29 degrees over the previous 3 months. The patient also had a lytic destruction of the C3 vertebral body with subluxation of C3-4.
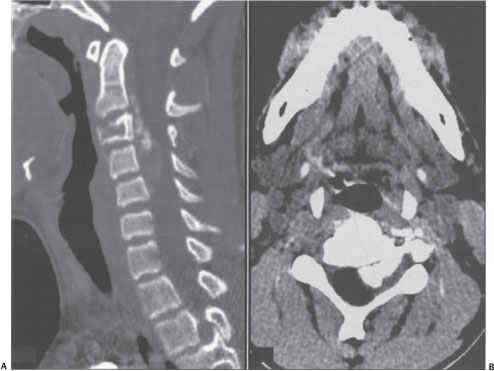
Figure 27–1 (A) Sagittal and (B) axial computed tomographic (CT) scan of a 12-year-old boy who had presented with a history of neck stiffness and weakness of the left upper limb. The CT scan done at the initial presentation shows an osteolytic lesion involving the vertebral body of C3 extending into the left C2-3 neural foramen with bone formation in the epidural space. The left-sided foramen has been expanded. The enhancement of the soft tissue is extending to C4-5 along the epidural space.
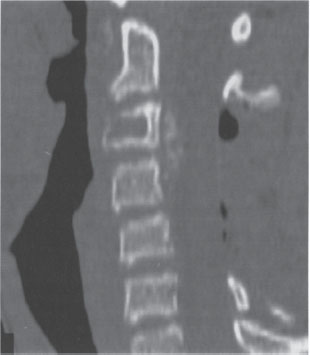
Figure 27–2 Postoperative sagittal computed tomographic scan after the patient underwent C3-5 laminectomy and excision of an extradural mass. The mass was later confirmed to be a Ewing sarcoma. He received chemotherapy and radiation therapy during the course of the follow-up.
On physical examination, the tone was normal. The motor strength of the upper and lower limbs was 5/5 bilaterally. Sensation was intact to light touch and pinprick in both upper and lower limbs bilaterally. Proprioception was intact. Deep tendon reflexes were absent for the biceps bilaterally, 2+ for triceps and brachioradialis bilaterally, and 1 + throughout the lower extremities bilaterally. Clonus was absent bilaterally. There were downgoing toes bilaterally. Hoffmann sign was negative.
Radiological Findings
A CT scan of the cervical spine at the time of presentation showed a kyphotic deformity centered at the C4 level with lytic destruction of the C3 vertebral body and subluxation of C3-4. Previous laminectomies of C3 through C5 were also demonstrated (Fig. 27–3).
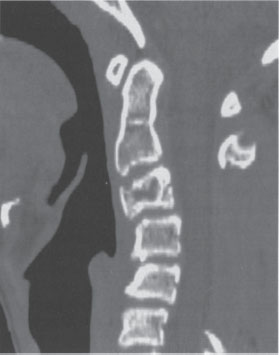
Figure 27–3 Sagittal computed tomographic (CT) scan performed 14 months postoperatively. The patient presented with complaints of neck pain and a flexed deformity. The CT scan shows a 29 degree kyphotic deformity from the inferior aspect of C2 to the inferior aspect of C5 centered at the C4 level with lytic destruction of the C3 vertebral body and a subluxation of C3-4. A previous laminectomy of C3 through C5 is also demonstrated.
Diagnosis
A diagnosis of postlaminectomy kyphosis with C3 fracture, subluxation of C3-4, and Ewing sarcoma was made based on the history, physical examination, and radiological findings.
 | Background |
The literature addressing postlaminectomy kyphosis of the cervical spine reveals significant variations in both the incidence and the preferred treatment. There are no prospective studies for this condition. All available information has been the result of retrospective reviews and anecdotal experiences.
The incidence of postlaminectomy kyphosis in the cervical spine is cited anywhere from 6 to 30% for adults and 9 to 100% for children. The 100% incidence in pediatric patients is a number that frequently appears in the literature to emphasize the increased risk for this deformity in skeletally immature patients. The 100% value occurred in a retrospective review out of Mayo Clinic in 1982 in which 26 patients under the age of 15 underwent laminectomies. Of the 26 patients, nine had cervical laminectomies and all nine developed kyphotic deformity within the 6-year follow-up period.1 More recent review articles have documented 38% of the Hospital for Sick Children in Toronto and between 37 and 95% quoted in a review from the Rothman Institute in Philadelphia.2,3
The literature considering preoperative risks for the development of postlaminectomy kyphosis tends to be more consistent than that regarding the incidence. Among the most frequently cited risk factors are preoperative loss of lordosis, facet capsule destruction, skeletal immaturity, and use of ionizing irradiation.2,4 The common thread for these risk factors is a compromise of the structural stability of the posterior column. To explain the increased incidence in the pediatric population, Yasuoka et al described a wedge deformity with pseudosubluxation of the facet joint.4 This widely accepted model attributes the increased incidence to the incomplete ossification of the vertebral bodies and the viscoelasticity of the ligaments in the pediatric population. The compressibility of the vertebral bodies and the decreased tensile strength of the posterior column favor kyphotic deformity in skeletally immature patients.
With the potential for such a high incidence of deformity, prevention has to be addressed. The two methods for prevention are use of laminoplasty versus laminectomy with fusion. One of the most comprehensive studies on laminoplasty was published in 2004.5 The authors concluded that there is “no benefit of laminoplasty over laminectomy with fusion in terms of preservation of spinal alignment, prevention of kyphotic deformity and neurological outcome.” This paper further states that complications such as “C5 radiculopathy and axial neck pain, although not unique to any technique, occurred at an alarming frequency in laminoplasty patients.” For this reason, the authors found laminoplasty suboptimal. This meta-analysis included 71 studies that amounted to a sample size of greater than 2000 patients. However, smaller studies have disagreed with the foregoing conclusions. An independent matched cohort clinical and radiographic retrospective analysis of laminoplasty versus laminectomy with fusion in 26 patients for multilevel cervical myelopathy concludes quite the opposite. The authors report that laminoplasty was superior to laminectomy with posterior fusion due to greater complications in the fusion group, including progression of myelopathy, pseudarthrosis, implant failure, subjacent degeneration requiring reoperation, development of moderate cervical kyphosis, deep infection requiring reoperation, and iliac autograft harvest site discomfort for greater than 1 year.6 Sani et al and Heller et al acknowledged a trend favoring laminoplasty but noted that there were no statistically significant differences in outcomes. In addition, there was a small sample size and the laminectomy patients had kyphotic deformities preoperatively, whereas the laminoplasty patients had lordotic postural deformities preoperatively.5,6 This is an important point because many surgeons consider preoperative kyphosis a contraindication to dorsal cervical spine surgery.
The common ground among authors is to only consider prophylaxis in patients who are at high risk for developing postlaminectomy kyphosis. Albert and Vaccaro recommend consideration of posterior fusion for patients with multiple level decompression, any facet resection, preoperative sagittal instability, anterior pseudarthrosis, and skeletal immaturity.2 Yasuoka et al reported that posterior fusion should not be done in children at the time of laminectomy but instead favor follow-up and anterior fusion as soon as deformity is noted.4 In practice, it is more reasonable to go for a fusion in a patient with multiple risk factors. The risk of progressive kyphosis needs to be weighed against the complications associated with fusion, including implant failure, infection, and degeneration of adjacent levels.
The treatment of an established deformity has also been disputed in the literature. A review article by Huckell, in 1998, favored attempting to reduce the kyphosis with traction preoperatively and ultimately treating with an anterior release and fusion.7 The authors did not favor posterior fusion for treatment due to the risk of pseudarthrosis secondary to the graft being under tension rather than compression. We also favor this approach, with traction followed by anterior decompression and fusion. Posterior fusion and stabilization may be added to situations where substantial instability is present. In agreement with Huckell, we believe that there is an unacceptably high failure rate when a postlaminectomy kyphosis is treated solely with a posterior procedure.
A retrospective study of 20 cases with mean age of 50 years and mean kyphosis of 38 degrees in 1994 concluded that the “combined technique of cervical traction, anterior decompression, and iliac fusion with anterior osseous synthetic plate fixation is a viable option for the treatment of symptomatic postlaminectomy kyphosis.”8 This study did not consider posterior fusion for treatment due to the failure of preoperative traction to reduce the deformity and relieve symptoms. The mean reduction of kyphosis was 8 degrees with traction and 28 degrees after anterior decompression. Postoperatively, two patients were placed in a halo for 3 months secondary to osteoporotic bone, four- to five-level fusions, and previous failure. Eighteen remaining patients wore a hard collar for 6 to 8 weeks. Follow-up radiographs at 1 month, 6 months, and 1 year revealed a mean postoperative residual kyphosis of 16 degrees.
A 1998 review article by Lowery and McDonough describes their preferred treatment for patients with myelopathy, without ankylosis, secondary to postlaminectomy kyphosis. They utilized maximal postural correction, anterior corpectomies, and strut grafting with a junctional buttress plate, followed by sequential posterior segmental fixation and facet fusion using morcellized autograft bone.9
Steinmetz et al in 2003 published a retrospective review of 10 patients with a mean age of 40 years and a mean kyphosis of 13 degrees preoperatively. They concluded that a ventral decompression and fusion, with intermediate vertebral body fixation, is optimal for correction of the kyphotic deformity.10 Of the 10 patients, all achieved a solid arthrodesis at the time of last follow-up. The mean angle of kyphotic correction was 20 degrees.
A review article by Eichholz and Ryken in 2003 concluded that an anterior approach with decompression, preferably via diskectomies versus corpectomies, fusion using autograft bone, and stabilization with anterior cervical plating is optimal.11 The authors noted that when corpectomy was necessary to achieve reduction, an anterior fibula strut graft or an autograft-filled cage is a reasonable option. In addition this publication notes “excellent fusion rates using a polyetheretherketone interbody spacer in conjunction with recombinant human bone morphogenetic protein.” The advantage of such materials includes avoidance of the morbidity associated with harvesting of iliac crest autograft. Of note, this use of bone morphogenetic protein (BMP) is not approved by the Food and Drug Administration (FDA). There have been numerous recent anecdotal reports of excessive soft tissue swelling following the use of BMP in the anterior cervical spine. In cases of long strut grafts and unstable cervical spines, Vaccaro et al recommended the use of posterior instrumentation, specifically for anterior procedures with greater than two-level corpectomies.12
Finally, a retrospective study out of Japan with 30 patients averaging 47.1 years with an average kyphosis of 29.4 degrees evaluated the effectiveness of pedicle screw fixation for correction of cervical kyphosis.13 The patient population was divided into two groups. Group I consisted of 17 patients, all of whom had flexible kyphosis. Group II included the remaining 13 patients, all with fixed deformities. Patients in Group I were treated with posterior correction alone. Group II patients all underwent both anterior and posterior correction during a single anesthesia. Posterior stabilization was achieved with standard pedicle screw placement, bone grafting with either or both local bone and chipped iliac bone and plate or rod application. Fixed kyphotic deformities of group II underwent circumferential osteotomy, including facetectomies, anterior corpectomy with uncinectomy, posterior correction with pedicle screw fixation, and anterior strut grafting. Of significance, only two patients in group I and one patient in group II had a diagnosis of postlaminectomy kyphosis. The two postlaminectomy patients in group I, a 22-year-old female and a 74-year-old female, both had 36 degrees of reduction in their kyphosis. The 74-year-old had improvement of her Frankel grading from a preoperative grade of C to a postoperative grade of D. One postlaminectomy patient in group II, a 36-year-old female, had 30 degrees of correction and improved from a Frankel grade of C to D. Of note, pedicle screw instrumentation is not FDA approved in the cervical spine, and careful review of a postoperative CT scan is essential to determine the efficacy of this procedure.
The majority of these studies cited were in the adult population. The pediatric population is at higher risk of postlaminectomy kyphosis due to weaker ventral bone and more lax dorsal ligamentous structures. For that same reason, it is our belief that preoperative traction is valuable and effective in this group of patients. Due to these anatomical issues, which favor the development of kyphosis, we will routinely fixate these patients anteriorly after traction is completed. The decision to perform a posterior stabilization procedure is made on a case-by-case basis. A greater number of levels decompressed, younger patients, and a large correction of deformity with traction are all factors that would tend to favor including a posterior surgery to achieve an anteroposterior (AP) stabilization and fusion.
 | Authors’ Preferred Method of Surgical Management |
The patient was admitted and taken to the operating room for a two-step procedure. First, the patient was positioned supine under general anesthesia. Gardner-Wells (Stealth Surgical Culver, IN) tong traction was applied and an anterior decompression with C3 corpectomy and C4-5 and C5-6 diskectomies and fusion of C2 through C6 were performed. Autologous, structural iliac crest grafting and screw-plate fixation was utilized. The patient was then placed in a halo and positioned prone for revision laminectomies of C3 through C5 and fusion of C2 through C6 with local bone and autologous morcellized iliac bone graft and screw-rod fixation (Fig. 27–4).
Pearls
The combined anterior-posterior approach is reserved for cases with involvement of more than two levels. The advantages of a combined approach include deformity correction via traction and the anterior approach and supplementation of the construct stability by the posterior approach. A compromised posterior tension band leads to substantial instability for which the combined approach has been advocated. The combined approach provides a rigid fixation to both the anterior and the posterior columns leading to decreased implant failure rates and higher fusion rates.



