Fig. 1
Sevoflurane preconditioning suppresses the apoptotic death pathway. (a) PANT staining and (b) immunofluorescence photomicrographs of cleaved caspase-3 (red) with Hoechst counterstaining (blue) of the infarct at 24 h after MCAO. Scale bar = 10 μm. (c) Counting of PANT positive and caspase-3 positive cell numbers, showing reduced apoptotic cell death after APC. ***p < 0.001 compared with control group. (d) Representative Western blot and semi-quantitation of cleaved ccaspase-3 in the brain at various time points after MCAO. **p < 0.01 compared with control group. (e) Representative Western blot and (f) semi-quantitation of cytochrome c in the brain at various time points after MCAO. *p < 0.05 compared with control group
Sevoflurane Preconditioning Increases the Ratio of Anti-apoptotic Proteins over Pro-apoptotic Proteins of the Bcl-2 Family
As shown above, sevoflurane preconditioning suppresses mitochondrial apoptotic pathways. We next detected whether sevoflurane preconditioning influences the ratio of anti-apoptotic proteins over pro-apoptotic proteins of the Bcl-2 family, the upper stream of the mitochondrial apoptotic pathway. As shown in Fig. 2, both anti-apoptotic proteins and pro-apoptotic proteins of the Bcl-2 family increased after ischemic insult; however, the levels of Bcl-2 and Bcl-xL increases were greater than the levels of Bax and Bid increases, indicating that sevoflurane preconditioning suppresses apoptotic death by regulating Bcl-2 family members.
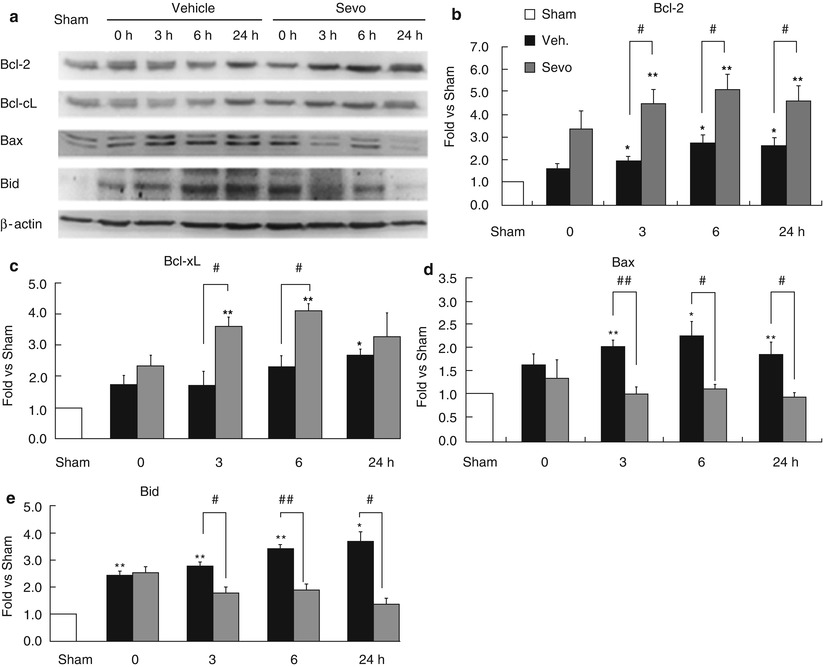

Fig. 2
Sevoflurane preconditioning enhances anti-apoptotic machinery. (a) Representative Western blots of Bcl-2 family members and semi-quantitative analysis of (b) Bcl-2, (c) Bcl-xL, (d) Bax, and (e) Bid levels in the cortex after MCAO, showing that sevoflurane preconditioning increased the ratio of anti-apoptotic proteins over pro-apoptotic proteins. n = 4; **p < 0.01, *p < 0.05 compared with sham group and ##p < 0.01, #p < 0.05 compared with vehicle group
Inhibition of JNK and p53 Was Involved in the Neuroprotection of Sevoflurane
Activation of JNK and p53 is a contributing factor in the pathophysiology of cerebral ischemia [23]. Therefore, we further investigated whether sevoflurane preconditioning inhibits these two pathways. We collected the cortical tissues and extracted the cytosolic and nuclear proteins and then subjected the proteins to Western blots. As shown in Fig. 3a, sevoflurane preconditioning significantly decreased the levels of p-JNK compared with the control; consequently, the levels of p-c-jun, a downstream molecule of JNK, were also reduced (Fig. 3b). Similarly, sevoflurane preconditioning also significantly decreased the levels of p53 nuclear translocation and Puma, one of its target gene products. Collectively, these findings suggested that the neuroprotection of sevoflurane preconditioning involves the inhibition of JNK and p53 pathways.
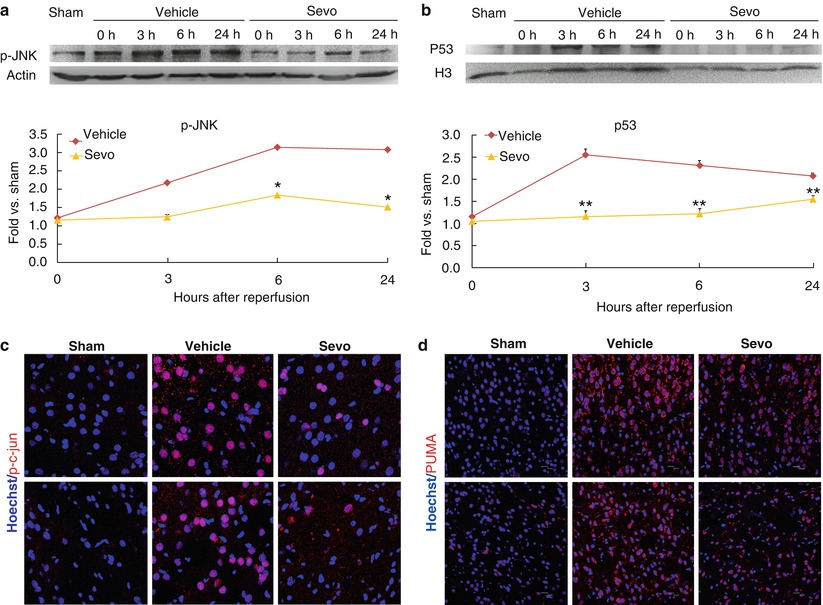

Fig. 3
Sevoflurane preconditioning attenuates the JNK and p53 pathways. (a) Representative Western blots and semi-quantitative analysis of p-JNK in cortex at various time points after MCAO. n = 4, *p < 0.05 compared with vehicle group. (b) Immunofluorescence photomicrographs of p-c-jun at 24 h after MCAO, scale bar = 20 μm. (c) Representative Western blots and semi-quantitative analysis of p53 in the nuclei at various time points after MCAO. **p < 0.01 compared with vehicle group. (d) Immunofluorescence photomicrographs of Puma at 24 h after MCAO, scale bar = 20 μm
Discussion
We have previously reported that repeated pretreatment with 1 MAC sevoflurane 24 h before MCAO exerts a neuroprotective effect [29]. In the current study, we further demonstrated that the repetitive preconditioning of sevoflurane markedly reduced apoptotic cell death, caspase-3 cleavage, and cytochrome c release. Sevoflurane preconditioning also robustly suppressed pro-apoptotic proteins and increased anti-apoptotic proteins of the Bcl-2 super family. Furthermore, APC with sevoflurane attenuated JNK and p53 pathways.
In agreement with previous reports [14, 15, 19], our finding also showed that apoptotic suppression plays an important role in APC. Although the exact regulative mechanisms remain unclear, influence on mitochondrial apoptotic pathways appears to be important [14, 15, 19, 21, 30]. For example, sevoflurane preconditioning inhibited mitochondrial permeability transition pore opening after cerebral ischemia [30], and desflurane preserved mitochondrial function after cerebral ischemia reperfusion injury [16]. In support, our data clearly demonstrated that sevoflurane preconditioning reduced cytochrome c release, a marker for the mitochondrial apoptotic pathway, further indicating that sevoflurane preconditioning largely inhibits mitochondrial apoptotic pathway.
One of the checkpoints of the mitochondrial apoptotic pathway is regulated by the balance between anti-apoptotic proteins and pro-apoptotic proteins of the Bcl-2 family [20, 22, 31]. Previous reports suggested that APC might regulate Bcl-2 family proteins. Sevoflurane exposure during 30 min MCAO in rats reduced the Bax level as early as 4 h after ischemic insult [15]. In a rat forebrain ischemia model, sevoflurane increased the levels of Bcl-2 and Mdm-2 and reduced the Bax level in the first 3 days after ischemia [32]. Sevoflurane preconditioning was also reported to increase Bcl-2 in rat brain following focal ischemia [19]. In this study, we systemically analyzed both anti-apoptotic and pro-apoptotic protein levels of Bcl-2 family and found that the overall effect of sevoflurane preconditioning was to increase the ratio of anti-apoptotic proteins over pro-apoptotic proteins of Bcl-2 family, therefore inhibiting apoptotic cell death after cerebral ischemia.
It is unclear how sevoflurane preconditioning influences the expression of Bcl-2 family proteins. Among the transcription factors that regulate the Bcl-2 family, c-jun and p53 are noticeable [23, 24, 33]. JNK pathway activates Bad and Bax [34–36]; JNK can also induce the expression of p53 [37]. The activation of p53 promotes apoptosis. In mice deficient in p53, Bcl-2 increases but Bax decreases in several tissues, suggesting a possible mechanism by which p53 regulates apoptosis [33]. In addition, p53 induces Puma, which interacts with Bcl-xL and promotes mitochondrial translocation and multimerization of Bax [25]. We observed that sevoflurane preconditioning inhibited JNK and p53 pathways, which could be contributing factors in sevoflurane preconditioning attenuation of apoptotic cell death after stroke.
In summary, we demonstrated that sevoflurane preconditioning protected the brain against brain ischemia and suppressed apoptotic cell death following MCAO in rats by regulating Bcl-2 family members. Sevoflurane preconditioning could be a useful method to induce ischemic tolerance.
Acknowledgments
This work was supported by NSFC 81301123 (to HW), 81171149, 81371306 (to YG), and 81228008 (to JC); the Shanghai Committee of Science and Technology Support Program 14431907002 (to YG); the Doctoral Fund of the Chinese Ministry of Education 20120071110042 (JC); National Institutes of Health/National Institute of Neurological Disorders and Stroke grants NS36736, NS43802, and NS45048 (to JC); and the Natural Science Foundation of Shanghai 14ZR1434600 (to HS).
References
1.
2.
Stetler RA, Leak RK, Gan Y, Li P, Zhang F, Hu X et al (2014) Preconditioning provides neuroprotection in models of CNS disease: paradigms and clinical significance. Prog Neurobiol 114:58–83CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








