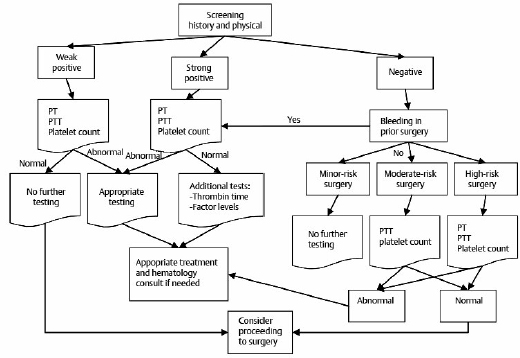
3 When a blood vessel is injured, the vascular, platelet, coagulation, and fibrinolytic systems interact in a finely orchestrated and coordinated fashion to prevent blood loss by localizing the thrombus to the site of injury. Bleeding can arise from abnormalities in any one, or a combination, of these four components of the hemostatic system. The physiology of this system is complex, and the current laboratory tests, which are widely used, cannot precisely reproduce these in vivo hemostatic processes.1 To evaluate bleeding tendencies, routine coagulation testing is often performed before surgical procedures. In most cases, this practice could reasonably be eliminated because it has been demonstrated that the best preoperative screen for bleeding disorders is a careful clinical history.2–5 Routine preoperative coagulation testing contributes little to patient care.6–9 Recently, the British Committee for Standards in Haematology1 published guidelines on the assessment of bleeding risk prior to surgery or invasive procedures. Based on a literature review, the committee found a poor predictive value of preoperative hemostatic tests, and thus its recommendation is that patients with a negative bleeding history do not require routine coagulation screening prior to surgery. However, this recommendation was based on a small number of studies. Also recently, the Italian Society for Haemostasis and Thrombosis (SISET: Società Italiana per lo Studio dell’ Emostasi e della Trombosi)10 published guidelines for the preoperative and preprocedural assessment of the bleeding risk, with the aim of reducing the incidence of preventable bleeding complications and limiting laboratory tests to those necessary. The areas with evidence (although of low quality) included neurosurgery in adults, and demonstrated the clinical value of a good history, prothrombin time (PT), partial thromboplastin time (PTT), and platelet counts. Studies were also evaluated in children undergoing major surgery, including adenotonsillectomy and neurosurgery, and invasive procedures. There were limitations to these guidelines because all retrieved studies were of low methodological quality with a high potential for bias, because they were not conducted using blinded outcome assessments. In addition, variable criteria for the severity of bleeding events and different normal reference values of the laboratory tests were adopted. A retrospective study of 1,211 patients undergoing neurosurgery procedures over a 1-year period was conducted at the Royal Melbourne Hospital, Victoria, Australia. Many patients’ history had clinical features indicating a potential bleeding tendency, but only a prolonged PTT, cranial surgery, and the use of antihypertensive and anesthetic drugs preoperatively predicted postoperative bleeding. Prolonged PTT was predictable based on the history in most patients.11 Preoperative platelet measurement is of questionable utility, as are all other routine laboratory screening tests.8 However, there is a relative consensus to perform this measurement before certain surgeries on highly vascular organs such as cardiac operations and neurosurgical procedures. A normal platelet count eliminates the need for concern about the common disorder of primary hemostasis—thrombocytopenia. This baseline value can also be useful in retrospect in evaluating perioperative or postoperative bleeding, or in the case of heparin-induced thrombocytopenia where a baseline platelet count is important for diagnosis. The PT is measured using an automated analyzer. It is used to assess the extrinsic and common pathways of clotting, which consist of tissue factor and factor VII, and coagulation factors in the common pathway (factors II [prothrombin], V, X, and fibrinogen). The international normalized ratio (INR) format should only be used as a standardized measure of PT evaluation for patients on warfarin or other vitamin K antagonists. The PTT is measured using an automated analyzer. It tests the integrity of the intrinsic and common pathways of coagulation. It detects deficiencies and inhibitors of the intrinsic (factors VIII, IX, and XI) and common pathway factors (including lupus anticoagulant and therapeutic anticoagulants). It also detects deficiency of factor XII, prekallikrein, and high molecular weight kininogen. Thus, the PTT detects hemophilias (deficiencies in factor VIII and IX) directly and von Willebrand’s disease indirectly. It tends to detect the more important and frequent disorders of coagulation. Hence, if only one test were to be performed to detect a disorder of coagulation, it should be the PTT rather than the PT.12 The thrombin time (TT) measures the final step of the clotting pathway—the conversion of fibrinogen to fibrin through the addition of exogenous thrombin to the patient’s plasma. It can potentially detect significant hypofibrinogenemia, dysfibrinogenemia, excessive fibrinolysis, and fibrin degradation products. One of the major causes of a prolonged thrombin time is heparin. The presence of heparin in plasma can be confirmed using the reptilase time assay. An enzyme derived from snake venom called reptilase is used instead of thrombin in the assay. Reptilase has an action similar to that of thrombin, but unlike thrombin it is not inhibited by heparin. A patient receiving heparin, therefore, would have a prolonged TT, but a normal reptilase time. These tests should not be performed routinely and should be ordered in consultation with a hematologist. Most of these tests have not been validated for use in the preoperative setting. Review of the blood smear by a hematologist or a hematopathologist would be helpful to evaluate the etiologies of thrombocytopenia or thrombocytosis. D-dimer is a fibrin degradation product (FDP), a small protein fragment present in the blood after a blood clot is degraded by fibrinolysis. It is so named because it contains two cross-linked D domains of the fibrinogen protein. In disseminated intravascular coagulation (DIC), there is evidence of fibrin breakdown such as elevated level of D-dimer and FDPs. Given the massive fibrin deposition that can occur in DIC, fibrinogen levels are usually decreased. However, this is not always the case because fibrinogen is an acute-phase reactant that is increased in inflammation, and although fibrinogen values may decrease as the DIC progresses, hypofibrinogenemia is not reliably found in all cases of DIC. The gold standard for diagnosing DIC is the D-dimer assay. Specific factor levels can be measured, including factor VIII, IX, and XI levels. This test is used to distinguish factor deficiency from factor inhibitor, such as the lupus anticoagulant, or specific factor inhibitors, such as antibodies directed against factor VIII. Mixing studies take advantage of the fact that factor levels that are 50% of normal yield a normal PT or PTT result. The test is done by mixing patient’s plasma 1:1 with normal plasma that contains 100% of all factor levels. Correction of the PT or PTT assay with mixing indicates factor deficiency; failure to correct indicates an inhibitor. This panel includes an assay for von Willebrand factor (vWF) antigen (vWF:Ag), which measures the amount of vW protein, and vWF ristocetin cofactor activity (vWF:RCo) which measures the function of the vW protein. Factor VIII is also a component of the vW panel. This test has been historically used to predict surgical bleeding. Although it may provide an estimate of platelet function, it is not a good screening test to predict bleeding risk. In one review of 13 studies (two of which were prospective studies) involving the bleeding time (BT) as a screening test, there was no correlation between the BT and surgical bleeding.13 Given that it is insensitive, invasive, time consuming, and subject to variation due to technical factors, it presently has a very minimal role in hemostasis testing, and consensus recommendations do not support using the BT test. This test has been reported to be superior to the BT as a screening test of primary hemostasis, and it has replaced the bleeding time for purposes of screening for von Willebrand disease (vWD) and intrinsic platelet hypofunction.14 However, a consensus panel of the International Society of Thrombosis and Hemostasis does not recommend use of the PFA-100 test due to the lack of studies validating its utility.15 This test has many technical requirements, including that the specimen must be received within 1 hour of phlebotomy, and that the patient must be fasting and not taking certain medications. The test is useful to evaluate patients with suspected inherited qualitative platelet disorders. This test may not be accurate if platelets are substantially decreased (<100,000/μL). Platelet aggregation is the gold standard for platelet function. Thromboelastography has been used as a technique for over half a century to assess global blood coagulation and fibrinolysis. The development of point-of-care analyzers has rekindled interest in this test for assessment of bleeding risk. The method measures the rate of fibrin polymerization and clot strength in plasma or whole blood as reflected in viscoelastic changes within the test sample.16 Some studies have validated the utility of this test in the setting of hepatic surgery, especially liver transplantation.17 A few studies have assessed the utility of perioperative assessment of coagulation in pediatric and adult neurosurgical patients using thromboelastography.18–20 In these studies, thrombelastograph coagulation analysis gave a hint to the development of a hypercoagulable state after surgery. However, based on the limited results of these small studies, routine use of thromboelastography in the clinical setting is not currently recommended. Coagulation screening tests can be meaningfully interpreted only with knowledge of their limitations and the relevant clinical situation. Most of these tests are in vitro laboratory assays that measure the time to clot formation in a test tube and that require the addition of exogenous reagents. Interpretation thus requires caution, as these tests may not accurately reflect the in vivo hemostatic response.1 Additionally in humans there are normal biological variations that need to be factored in. In laboratory practice, the normal range is usually derived from disease-free subjects and defined as results that fall within two standard deviations above and below the mean for the normal population. Therefore, by definition, 5% of healthy subjects would have an abnormal coagulation test. In the absence of relevant clinical information, unnecessary further investigations may thus be prompted, generating delay, anxiety, cost, and perhaps unnecessary exposure to blood products. There are also technical variations in the method of performing the tests, as well as difficulties such as artifacts due to prolonged tourniquet placement, difficult or traumatic phlebotomy, inadequate sample volumes, heparin contamination, prolonged storage, and sampling from a line. Pathological states and clinically important diseases may be modified or masked by physiological responses. For example, factor VIII levels rise markedly in pregnancy and in response to physical stress and trauma. This results in a shortening of the PTT, which may mask the detection of mild hemophilia A and vWD. The excellent review published by Samuel Rapaport5 in 1983, “Preoperative Hemostatic Evaluation: Which Tests, If Any?” continues to be the basis of our recommended approach. This approach to the general surgery patient would also be applicable to neurosurgical patients. Important clinical history questions to ask would include the following: 1. Is there a history of a bleeding disorder present? (This patient questionnaire has been adapted from Rapaport’s5 review and Koscielny et al’s21 review.) • Do you experience excess bleeding in your mouth/gums or frequent nosebleeds without apparent reason? • Have you bled into a muscle or a joint? Have you ever had blood in your stool? • Do you develop large bruises or “blue spots” (hematoma) even in the absence of obvious injury/trauma? Have you bled excessively after small wounds? If so, how often do you have bleedings or “blue spots” (hematoma): one or two times a week or more often? • Did you ever have prolonged or grave bleedings during or after a tooth extraction? • What operations have you undergone, including minor procedures such as skin biopsies or colonoscopy/bronchoscopy with biopsies? Was there any bleeding, either immediate or delayed? • Do you have other medical problems? Is there a history of hepatic, renal, or hematologic disease? Have you ever required a transfusion of whole blood, red blood cells, platelets, plasma, or blood clotting factors? If so, please list the operation(s) or the reason(s). • Do you have profuse menstrual bleeding? Do you have the impression that you have prolonged menstruation (> 7 days) or a high frequency of tampon change? 2. If there is a bleeding history, is the disorder familial or acquired? • Age at presentation • Duration of symptoms • Response to previous hemostatic challenges • Family history: Do any relatives have bleeding tendencies or experience excessive bleeding following surgery? 3. What type of bleeding disorder is present? Table 3.1 summarizes clinical and physical examination features that are helpful in distinguishing platelet-type bleeding disorders from coagulation-type bleeding disorders. 4. Is there an underlying systemic disease? These would include: • Liver disease • Can cause thrombocytopenia (congestive splenomegaly) • Increased fibrinolysis (deficiency of liver-derived factors that inhibit fibrinolysis, e.g., α2-plasmin inhibitor) • Dysfibrinogenemia • Renal impairment • Hypothyroidism • Amyloidosis • DIC: infection, trauma, tumors, toxins, obstetric complications, metabolic disorders • Drug history • Pregnancy Table 3.1 Characteristics of Platelet- and Coagulation-Type Bleeding
Preoperative Coagulation Assessment for the Neurosurgical Patient
Evidence-Based Background
Common and Uncommon Coagulation Tests
Common Tests
Platelet Count
Prothrombin Time
Activated Partial Thromboplastin Time
Thrombin Time
Uncommon Tests
Peripheral Blood Smear
Disseminated Intravascular Coagulation Panel: D-Dimer, Fibrinogen, Fibrin Degradation Products
Factor Activity Levels
1:1 Mixing Study
von Willebrand Panel
Bleeding Time
Platelet Function Analyzer (PFA-100)
Platelet Aggregation Studies
Thromboelastography
Limitations of Coagulation Testing
Recommended Assessment Strategies for Routine Patients
Clinical Assessment
Platelet-Type bleeding | Coagulation-Type Bleeding |
Mucocutaneous | Large soft tissue bruises |
Bleeding after minor cuts | Not usually |
Small, superficial ecchymoses | Large, palpable ecchymoses |
Petechiae and purpura | Hemarthrosis |
Dominant family history | X-linked recessive history |
Female predominance | Male predominance |
Early bleeding/oozing, relatively milder to moderate | Delayed bleeding, relatively moderate to severe |
Note: This table summarizes the features on history and physical exam that could help to distinguish bleeding arising from platelet deficiency/dysfunction versus defects in the coagulation cascade.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree