Fig. 14.1
Gadolinium-enhanced axial image showing avid enhancement of a right middle fossa meningioma (a). Catheter angiography demonstrates a significant tumor blush with microcatheter injection of the internal maxillary artery on anterior–posterior (b) and lateral (c) projections. No residual enhancement is seen on intraoperative gadolinium-enhanced MRI (d)
Posterior fossa meningioma
Posteromedially located posterior fossa meningioma often receives supply from meningeal branches of the vertebral arteries. More laterally located posterior fossa meingiomas commonly receives supply from transmastoid branches of the occipital artery as well as contribution from the ascending pharyngeal artery. Preoperative embolization of meningioma feeding arteries is usually confined to large tumors with high vascularity. Evidence of reduction in perioperative transfusion associated with embolization has been reported [48].
Juvenile Nasopharyngeal Angiofibroma
Juvenile nasopharyngeal angiofibroma (JNA) is a benign tumor that tends to bleed and occurs in the nasopharynx of prepubertal and adolescent males. It originates from the posterolateral nasopharynx in the pterygopalatine fossa and extends through the sphenopalatine foramen to involve both the pterygopalatine fossa and the posterior nasal cavity [49]. It may extend laterally through the pterygomaxillary fissure into the infratemporal fossa. Anterior bowing of the posterior wall of the maxillary antrum is a result of remodeling and expansion of the bony wall of the pterygopalatine fossa. Occasionally, the greater wing of the sphenoid may be eroded, exposing the middle fossa dura. Sphenoid sinus involvement through its ostium may afford access into the medial portion of the middle fossa. Orbital extension via the inferior orbital fissure may occur in approximately one third of the patients [50].
Arterial supply for JNA arises initially from the pterygopalatine portion of the internal maxillary artery including the sphenopalatine, infraorbital, and descending palatine branches. Recruitment of adjacent vessels including the accessory meningeal, ascending pharyngeal, and ascending palatine arteries is common with larger tumors. Pial supply from the ICA, although uncommon, may exist, reflecting tumor extension into the anterior or middle fossa [51]. Bilateral blood supply is not uncommon, and when present, both branches should be embolized to prevent excessive hemorrhage at the time of resection [52].
Complete surgical resection is the therapy of choice. Preoperative embolization of JNA has been shown to reduce both perioperative blood loss and the duration of surgical resection [53]. Preoperative embolization has typically been performed via a transarterial route using a variety of embolic materials.
The JNA location and routes of extension mandate a particular attention to the possibility of orbital or intracranial anastomoses. Supply to cranial nerves is also of concern when embolization is attempted for tumor involving the skull base [53]. Endoscopic assistance has been used for direct transnasal tumor puncture and intratumoral embolization using the liquid embolic agent Onyx [54].
Illustrative Case 2: Juvenile Nasal Angiofibroma
A 22-year-old man who presented with recurrent epistaxis was found to have a right maxillary sinus mass on MRI (Fig. 14.2a). A biopsy confirmed the diagnosis of JNA. The patient underwent diagnostic angiography revealing splaying of the right internal maxillary artery and tumor blush (Fig. 14.2b). The patient underwent successful embolization with PVA particles (Fig. 14.2c, d) followed by complete resection.
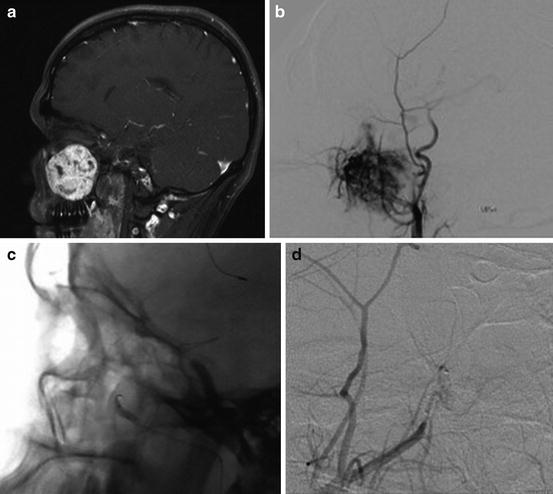

Fig. 14.2
Gadolinium-enhanced sagittal MRI showing avid enhancement of a maxillary sinus juvenile nasal angiofibroma (a). Microcatheter injection of the internal maxillary artery demonstrates significant tumor blush (b) that was treated with particle embolization (c), resulting in elimination of the previously seen blush (d)
Glomus Tumors
Paragangliomas, also known as glomus tumors, are rare hypervascular neoplasms that arise from chemoreceptor organs derived from the neural crest [55].Carotid body location is the most common. Temporal bone paragangliomas are next in frequency and include glomus tympanicum tumors that involve the middle ear and are associated with the tympanic branch of the glossopharyngeal nerve. The glomus jugulare tumors involve the jugular fossa and thought to originate from the chief cell located within the jugular bulb adventitia. Paraganglioma associated with the auricular branch of the vagus nerve (glomus vagale) and those involving the larynx are less common [56].
Glomus tumors are found most commonly in women during the fifth and sixth decades. Wide age distribution is reported with earlier onset in familial cases. Due to the tumor’s slow growing nature, the clinical presentation is often indolent and delayed. Clinical manifestations are usually related to the location of the tumor and the infiltration of adjacent structure. While the majority of paragangliomas show histological evidence of catecholamine production, clinical hypersecretion occurs in less than 5 % of cases. Hypersecretion of catecholamines can result in paroxysmal hypertension, headache, nausea, and excessive perspiration [57].
CT scans of the skull base will usually demonstrate the extent of bone thinning or bone erosion around the mass lesion. MR images are the mainstay of noninvasive images and typically demonstrate the characteristic “salt-and-pepper” appearance of high-velocity flow voids within the tumor. Complete surgical resection, usually with preoperative embolization of major external carotid artery feeding arteries, is the mainstay of therapy [58]. Preoperative embolization of the ECA supply usually gives the most favorable risk–benefit ratio for the vast majority of paragangliomas of the head and neck [59]. Balloon occlusion testing of the ICA may be necessary when carotid encasement is present or carotid sacrifice is anticipated. Superselective angiography is important to delineate cranial nerve supply or dangerous anastomoses with the intracranial circulation. Jugulotympanic paragangliomas receive major supply from ascending pharyngeal artery. Middle ear paragangliomas receive their blood supply from the inferior tympanic branch while the neuromeningeal branch supplies both the jugular fossa and hypoglossal canal. Additional ECA supply from the temporal branch of the middle meningeal artery, transmastoid branches of the occipital artery, or extradural ICA (caroticotympanic or cavernous branches) may contribute to the tumor blood supply. The musculospinal branches of the ascending pharyngeal artery supply vagal paraganglioma inferior to the skull base [60].
Hemangiopericytoma
The term hemangiopericytoma (HPC) was first described by Stout and Murray in 1942 as a distinct neoplasm of pericytic origin. The cell of origin is believed to be the pericyte of Zimmerman, a modified smooth muscle cell that is found in the capillary wall. Up to 25 % of HPCs involve the head and neck with the sinonasal region being the most common location [61]. HPC intracranial involvement should be considered in the differential diagnosis of tumors involving the dura. They are most often supratentorial although posterior fossa location has been reported [62].
Typically presenting in the fifth and sixth decade of life, the tumor has been found in all age groups and affects both sex equally. Despite an apparently benign initial presentation, a propensity for local aggressiveness and metastases frequently characterizes HPC, leading to significant mortality rates [63].
Angiography often demonstrates high vascularity and may be considered when HPC is a differential consideration. Preoperative embolization has been recommended to aid in achieving a gross total resection [64].
Endolymphatic Sac Tumors
Endolymphatic sac tumors, first described in 1989 by Heffner et al., are rare lesions affecting females more commonly than males with a mean age of symptom onset in the fourth decade [65].They most often occur sporadically; nevertheless, they have also been described as a component of von Hippel–Lindau syndrome.
The tumors arise from the endolymphatic sac that is located in the bony vestibular aqueduct. It communicates with the endolymphic duct that in turn joins the utricular and saccular ducts. It is believed to be involved in the regulation of endolymphatic fluid volume [66].
CT scan will demonstrate an expansive, erosive soft tissue mass involving the temporal bone adjacent to the vestibular aqueduct. MR typically demonstrates a heterogeneous signal as a result from component of hemorrhage, cyst, residual bone, and cholesterol granuloma. Flow voids may be present suggesting high vascular flow [67].
The vascular nature of the neoplasm has led to the recommendations for preoperative embolization to aid in surgical resection [68].
References
1.
3.
4.
Djindjian R, Cophignon J, Rey Theron J, Merland JJ, Houdart R. Superselctive arteriographic embolization by the femoral route in neuroradiology. Study of 50 cases. Neuroradiolgy. 1973;6(3):143–52.CrossRef
5.
6.
7.
Manelfe C, Lasjaunias P, Ruscalleda J. Preoperative embolization of intracranial meningiomas. AJNR Am J Neuroradiol. 1986;7(5):963–72.PubMed
8.
9.
10.
Bendszus M, Martin-Schrader I, Schlake HP, Solymosi L. Embolisation of intracranial meningiomas without subsequent surgery. Neuroradiology. 2003;45(7):451–5.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








