, Damien Gervasoni1 and Catherine Vogt2
(1)
Lyon Neuroscience Research Centre CNRS UMR 5292–INSERM U 1028, Université Claude Bernard Lyon 1, Lyon, France
(2)
Université Claude Bernard Lyon 1, Lyon, France
5.1 General Preoperatory Conditions
5.1.1 Assessing the General Condition of Animals on the Day Before and on the Day of Surgery
Before any surgical procedure, it is important to ensure that the animals are in good health by checking their appearance and analyzing their general behavior. A clinical examination may be required to detect certain anomalies and to bring these to the attention of the investigator. In a neurosurgical procedure, it is necessary to eliminate any animal with an abnormality that can cause anatomical or behavioral alterations and thus influence the experimental results (Appendix 1). Signs of reduced appetite; weight loss; deficits in normal exploratory behavior; hyperresponsiveness to handling; vocalization; self-mutilation; prostration; bite marks; scratches; patchy, dull, and/or ruffled fur; and unusual posture or facial expression should immediately exclude the animal from the procedure. Any of these signs (Appendix 3) should be noted on the animal’s follow-up register (Appendix 2B) and reported to the veterinarian to determine the corrective course of action, treatment, prophylactic measures, or eventual euthanasia (Appendix 4).
5.1.2 Preparation of Surgical Equipment
The investigator responsible for the experiment and the surgical procedure must ensure the availability of the required equipment and its maintenance, preparation, and sterilization. A sufficient amount of time must be given to do all of this in advance, so as not to be caught short at the last moment (Appendix 5). The availability of the required anesthetics and their expiration dates should be verified. The laboratory stock and the administration of any anesthetic should be recorded in a register. This register is maintained by the laboratory personnel in charge of the pharmacy service within the institution of experimentation.
Benchtop instruments, including a cold light source and dental drill handpiece, are placed on the operating table next to the stereotaxic apparatus, along with a receptacle, such as a surgical kidney dish or plastic container, for organic waste (Figs. 5.1 and 5.7). A surgical box containing the sterilized surgical instruments (Figs. 5.2 and 5.7) is placed on a trolley in the clean zone (Fig. 5.8). From our experience, it is necessary to have a minimum of instruments such as those shown in Fig. 5.2 in order to carry out an incision of the head. A surgical box containing the suturing instruments (Figs. 5.3 and 5.7) is kept on a sterile surgical tray pending their use at the end of the surgery.
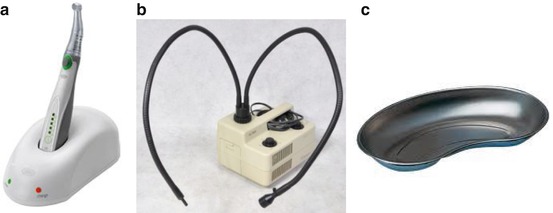
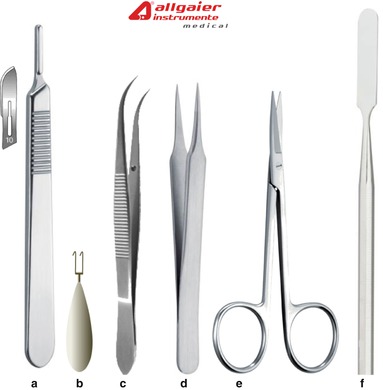
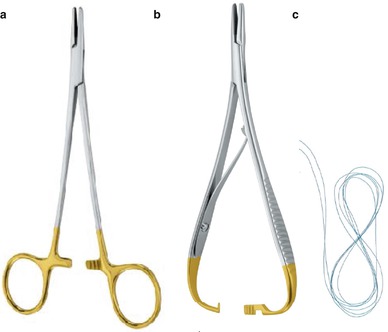

Fig. 5.1
Benchtop instruments. (a) Dental drill with angled handpiece, for piercing the skull bone at the time of the insertion procedure. Inserted material (cannula, electrode, etc.) must be chemically sterilized prior to the procedure. (b) Cold light source (double gooseneck fiber optic lamp). (c) Surgical kidney dish for disposal of organic waste

Fig. 5.2
Surgical instruments necessary for the incision procedure. (a) Scalpel handle and blade for skin incision, (b) Ferry tissue retractor prototype that will be manufactured by ALLGAIER Instrumente Medical. (c), (d) Straight and curved Dumont forceps for gripping the tissue. They are also used for grasping the microscrews and implantation materials. Excessive force should not be applied to avoid deforming the forceps jaws. (e) Fine surgical scissors. (f) Spatula for the preparation of dental cement

Fig. 5.3
Suturing instruments. (a) Standard needle holder Allgaier Instrumente Medical (b) Mathieu-type needle holder ALLGAIER Instrumente Medical. (c) Suture thread with attached needle as found in its sterile blister package
The rigorous undertaking of a surgical procedure requires the an assistant for the successive opening of different storage boxes containing the surgical drapes, instruments, disposable materials, and suture blister packs so that the gloved surgeon can access them without breaking the chain of asepsis. Sterilized disposable materials, cotton swabs, needles, and sutures (Fig. 5.4) should be placed on the sterile tray next to the box containing the surgical instruments. A sterile drum containing the operating drapes and gauze are placed nearby on a trolley in the “clean” zone (see Fig. 5.8). Surgical caps, masks, gowns, and gloves are made accessible to the operator to facilitate an aseptic procedure. Tips on the selection of suture material are provided in Fig. 5.4 and Table 5.1.
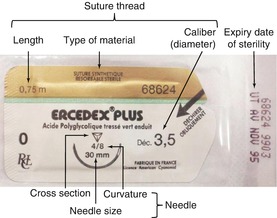
Table 5.1
Numbering codes and corresponding diameters of various suture threads
Threads for experimental surgery (rodents) | ||||||
|---|---|---|---|---|---|---|
Threads of microsurgery | Threads of general surgery | |||||
Caliber (diameter) n° Metric n° | 0.4 | 0.5 | 0.7 | 1 | 1.5 | 2 |
Commercial n° UPS (US pharmacopoeia) n° | 8/0 | 7/0 | 6/0 | 5/0 | 4/0 | 3/0 |
Diameter in microns | 45 | 60 | 85 | 125 | 175 | 225 |

Fig. 5.4
Example of a commercially available prepackaged suture
5.1.3 Sterilization of Surgical Equipment
Complications of infection related to a surgical procedure is a permanent risk in animal surgery. Sterilization of the surgical equipment is the first element in preventing postoperative infection. Two sterilization methods are commonly used.
Heat sterilization, since beyond a certain temperature, all life is impossible. Microorganisms, such as heat-resistant bacterial spores, are destroyed by heating for 30 min at 170 °C. This method can be used for heat-resistant material, including the implant materials (cannulae, electrodes, obturators) and the special equipment and tools used for drilling the skull bone. It can be performed using either an autoclave (Fig. 5.5), which ensures stability and a traceability of a recorded temperature curve, or even a domestic oven, provided that the heating conditions are increased (45 min at 170–180 °C). Cloth surgical drapes, gowns, and compresses can also be heat-sterilized in a drum (Fig. 5.6).

Fig. 5.5
Benchtop autoclave (18 l capacity, Webstore Medical®) with a compact footprint (25 × 45 cm)

Fig. 5.6
Examples of sterile drums (Covéto®)
Sterilization by immersion in an antiseptic solution can be used as an emergency workaround option. The material is immersed in an antiseptic solution for a length of time recommended by the manufacturer, usually around 10 min. Glutaraldehyde-based products containing corrosion inhibitors (Korsolex®, IS Mucadont®) offer good results, provided that the instruments are rinsed with sterile water before use.
It is important to separate the specialized (and often expensive) equipment from the surgical instruments and materials needed at hand during the operation. Two sets of instruments for the incision and suturing (scalpel, dissecting forceps, drill bits, scissors, and needle holder) allow the cleaning and sterilization of the first set used on animal “X,” while the second set is being used on animal “X + 1.” Special equipment that usually has little or no contact with the tissues of the anesthetized animal can be kept sterile by placing it in a tray covered with a sterile drape between each procedure (Fig. 5.7). The ear and incisor bars can be autoclaved or cleaned simply with a disinfectant towelette.
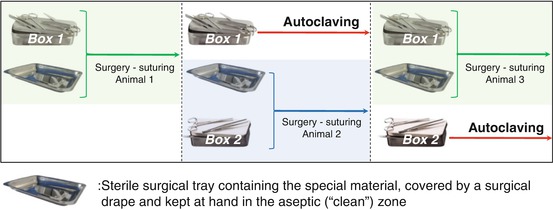

Fig. 5.7
Example of using two boxes of instruments during the same surgical procedure
5.1.4 Organizing and Managing Surgical Asepsis
A coherent organization of a surgical room, operating theater or a lab benchtop space used for surgery, is crucial to carrying out sterile surgery and limiting the risk of breaking the chain of asepsis. The principle of an operational workflow (a forward moving sequence of steps) commonly used in industrial sectors helps in organizing the different levels of cleanliness or sterility required at each stage of the procedure (Fig. 5.8). This organization identifies a “dirty” preparation zone in which shearing of the animal and induction of anesthesia is carried out and an “intermediate” zone where the paws of the animal are washed with antiseptic soap before bringing it into the “clean” zone on a thermostated electric heating blanket or pad equipped with a rectal temperature probe, just prior to being fixed in the stereotaxic apparatus.
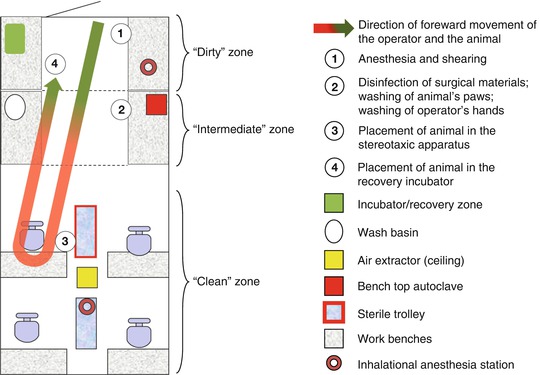

Fig. 5.8
Organization of a surgery room. The arrow represents the forward moving sequence of steps in the surgical procedure
After the disinfection of the top of the head (with soap, rinsing, 70 % ethanol, and a disinfectant such as chlorhexidine or polyvinylpyrrolidone iodine1), a subcutaneous injection of local anesthetic (see Sects. 5.2.3 and 5.2.4) is made on each side of the line of incision. This helps to limit the postoperative pain of the animal. Precision in carrying out the surgical procedure is important for limiting pain, morbidity, and mortality, hence, the importance of proper training in neurosurgical practices.
The first package of sutures opened by the assistant is put it on the surgical tray so the surgeon can take the sterile part of the package and use it at the end of the surgery for the suture.
Each micrometer screw of the stereotaxic apparatus is then disinfected, as well as the handpiece of the dental drill. With the aid of an assistant, the operator then dons a gown and mask from the sterilization drum (see Fig. 5.6), as well as sterile gloves. At this point, the operator proceeds with the set up of the sterile zone with help from the assistant who passes to him the different open boxes of materials. A sterile drape is spread to create the operating field; the different surgical instruments, compresses, and other consumables, as well as any drapes required for the animal, are then successively placed upon the drape. The first package of sutures opened by the assistant is put on the surgical tray so the surgeon can take the sterile part of the package and use it at the end of the surgery for the suture.
During successive procedures, equipment and materials are covered with a sterile drape while the second animal is being prepared and then reused once the operator has changed gloves. Before the skin incision, the operator makes a fresh disinfection of the operating zone 2 with chlorhexidine or polyvinylpyrrolidone iodine and then puts the surgical drapes in place.
At the end of the surgical procedure, the animal is placed in the recovery zone, for example, in an incubator or under a heating lamp in the preparation zone. This allows the operator to proceed, if necessary, with an operation on a second animal while monitoring the recovery of the first.
Ideally, all soiled instruments are cleaned or left to soak in the appropriate product in the “intermediate” zone. Once rinsed and dried, they can be autoclaved before reuse. For the second animal, the operator proceeds as before with the shearing and anesthesia in the preparation zone and then the setup of the animal. Normally, the entire procedure of dressing is repeated, in particular a change of surgical gowns, but it is common to limit this to a simple change of gloves.
5.2 Pain and Its Pre-, Intra-, and Postoperative Treatment
5.2.1 Definition
According to the International Association for the Study of Pain, pain is an “unpleasant sensory or emotional experience caused by actual or potential tissue injury… that provokes protective motor and autonomic reactions leading to a change in the behavior specific to the individual. The inability to communicate does not exclude the possibility that the subject feels pain and requires adequate analgesic treatment.” At the end of the twentieth century, a British survey of 1,000 practicing veterinarians revealed that only half of the animals undergoing an ovarian hysterectomy and two-thirds of those having abdominal surgery received an opioid analgesic or anti-inflammatory drug during their operation. Nearly one surgeon in 10 felt that “the surgical operation in question did not cause enough pain to warrant treatment.” Within the last decade, attitudes have changed significantly – the entire scientific community agrees that pain, if left untreated, increases the risk of morbidity and mortality and constitutes a significant bias for experimental results.
Pain in animals is difficult to assess because the subject is unable to directly communicate what it feels. Only those signs that are secondary to painful phenomenon are expressed and can thus be observed and treated retrospectively. It is nevertheless essential to anticipate and consider it and to deal with it more effectively. This is not only a moral issue but also a scientific and regulatory requirement. As a precautionary principle, it is considered that any procedure perceived as being painful in humans be regarded as such in animals and that a pretreatment thereof with diverse agents (e.g., opioids, steroidal or nonsteroidal anti-inflammatory drugs, local anesthetics, alpha-2 adrenergic receptor agonists) should be implemented. A review of the anatomical mediators of pain transmission is useful here for understanding the concept of more effective multimodal analgesia, which can allow the use of lower doses of drug and thus a reduction in the incidence of their side effects.
5.2.2 The Physiology of Pain: From Transduction to Perception
Nociception, or pain, is a physiological process that involves five steps: transduction, transmission, modulation, projection, and perception of a painful signal.
Transduction is a process by which nociceptors, specialized free nerve endings that are sensitive to mechanical, chemical, or thermal stimuli, convert these stimuli into signals (Table 5.2) that are relayed by neurons to higher centers. Nociceptors are present in the skin, muscles, joints, bones, and viscera.
Table 5.2
Different types of stimuli and the pain associated with them
Site | Type of stimulus | Example |
|---|---|---|
Skin | Thermal | Burn |
Mechanical | Cut | |
Chemical | Inflammation | |
Muscle | Mechanical | Blow, compression |
Chemical | Inflammation or intra-articular injection of irritant |
Peripheral sensation is transmitted by three types of nerve fibers: Abeta, Adelta, and C. Under normal conditions, the Abeta fibers carry non-painful sensory information such as friction, vibration, pressure, or proprioception. Nociceptors on Adelta and C fibers generate painful sensory information that is carried by the Adelta and C fibers to the gray matter in the dorsal horn of the spinal cord. This process is referred to as transmission (Fig. 5.9).
Within the spinal cord, a synaptic relay allows for a local modulation of the nociceptive signal by the intervention of three types of postsynaptic neurons:
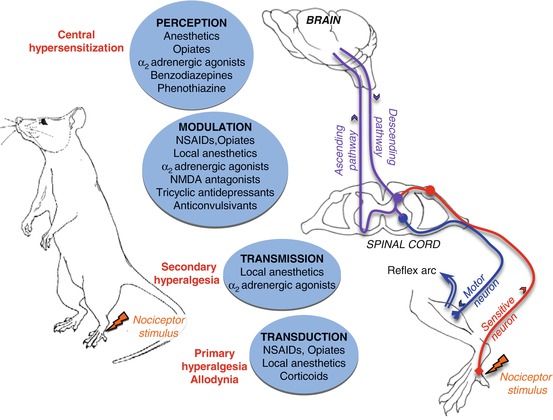
Modulatory interneurons responsible for local signal modulation.
Motor neurons responsible for the neural reflex arc.
Neurons of the ascending pathways that participate in directing the nociceptive signal towards centers of integration in the brain (thalamus, hypothalamus, and reticular formation) by way of the spinothalamic, spinoreticular, or trigeminal tracts. This process is called projection. The integration of the pain signal by cortical structures is referred to as perception.

Fig. 5.9
The major pathways of nociceptive information processing
Nociception: a physiological process by which pain signals are routed from pain receptors towards the brain. Nociceptors: pain receptors. Nociceptive stimuli: phenomena capable of activating nociceptors and generating a painful response. NSAIDs : nonsteroidal anti-inflammatory drugs
5.2.3 Amplification of the Pain Signal by Sensitization
Regulatory mechanisms are continuously solicited and established by the organism. A nociceptive signal can be amplified by the phenomena of peripheral and central sensitization. While discussed individually here for a better understanding, peripheral and central sensitizations are intricate and inseparable mechanisms (see Fig. 5.9).
Peripheral sensitization is related to a release of endogenous substances, such as inflammatory mediators, potassium, and ATP, into the local environment of the nociceptors in response to tissue damage and/or inflammation. This sensitization provokes a peripheral hyperalgesia, an exaggerated response to a noxious stimulus in the immediate vicinity of the lesion, and allodynia, an increase in the reactivity of nociceptors to stimuli that are not painful under normal conditions.
Peripheral sensitization can be partially treated by the intravenous administration of nonsteroidal anti-inflammatory drugs (NSAIDs). These agents inhibit the production of certain mediatory substances, but other endogenous compounds not directly involved, such as ATP and substance P, continue to have a sensitizing effect.
Central sensitization involves the synaptic relay in the spinal cord. Repeated and intense noxious stimuli cause temporal summation or windup. This activates NMDA (N-methyl-d-aspartate) receptors and lowers the threshold of excitability of postsynaptic neurons, resulting in a sensitization of regions several centimeters distant from the injured area, otherwise referred to as central hyperalgesia. In this context, nerve fibers that usually convey non-painful sensory messages transmit a modified signal that can be interpreted as painful. This is called allodynia.
5.2.4 Optimized or Multimodal Analgesia
“Anesthesia or the use of a sedative, even a powerful one, is not synonymous with analgesia” (Smith et al. 1985). The knowledge and understanding of painful phenomena has led to the development of effective strategies for analgesia. Multimodal analgesia aims to act at different stages of pain signal propagation, based on the combined use of compounds from different drug classes and/or different methods of administration, e.g., from intravenous for rapid and widespread onset and action to epidural or local injection for direct action on nerve fibers. These combinations exploit the complementarity and the eventual synergy between agents, permitting the use of lower doses and thus reducing the risks of adverse side effects (Shavit et al. 2005).
Commonly used dosages of the major analgesic drugs are presented in Appendix 7.
The most commonly used agents for blocking conduction in peripheral nociceptive fibers are the local anesthetics (2 % lidocaine and 0.5 % bupivacaine), which act by inhibiting the operation of sodium channels. They can be injected directly along specific nerve fiber projections (e.g., brachial plexus, cranial and intercostal nerves) or at the level of the nerve root(s) (e.g., epidural injection). Opioids, such as morphine, can also be administered by the epidural route.
5.2.4.1 Analgesic and Anti-hyperalgesic Effects of Lidocaine (from Joris 2008)
Lidocaine, administered by local or regional routes, has analgesic properties. Analgesia is exerted by specific blockade of tonic discharges in peripheral neurons evoked by nociceptive stimuli (Tanelian and MacIver 1991) and by increasing the excitation threshold of A∂ and C nerve fibers (Wallace et al. 1996). A direct action on nociceptive transmission at the spinal level has also been described (Nagy and Woolf 1996). Lidocaine blocks sodium channels (Kuno and Matsuura 1982), resulting in a decrease of postsynaptic depolarization triggered by the activation of neurokinin and NMDA receptors and a consequent reduction in the generation of hyperalgesia and allodynia. In animals, secondary nociceptive responses to visceral stimuli, rectal distension, and visceral motor and cardiovascular reflexes are also inhibited by lidocaine. The analgesic effect is dose dependent, attained at plasma concentrations of 2 μg/ml, with low interindividual variability (Ferrante et al. 1996). In addition to its analgesic effect, lidocaine is anti-hyperalgesic (Koppert et al. 2000).
Local anesthetics, NSAIDs, alpha-2 adrenergic receptor agonists (e.g., medetomidine), NMDA receptor antagonists (e.g., ketamine), tricyclic antidepressants, and anticonvulsants have actions at the level of modulation.
Opioids and alpha-2 adrenergic agonists are the major molecules, which, upon intravenous injection, act upon the synaptic relay of pain signals at the level of the dorsal horn in the spinal cord. These agents act on different types of receptors in the pre- and postsynaptic zones. When used in combination, they have a synergistic effect. The central mode of action of NSAIDs is not well known, but several studies have shown that in healthy animals (i.e., in the absence of inflammation), NSAIDs increase the threshold to nociceptive simulation. This effect is blocked by co-treatment with naloxone and with atipamezole, a selective alpha-2 receptor antagonist.
Opioids and alpha-2 adrenergic agonists reduce the perception of pain by acting on pain signal projection and the thalamic-cortical structures involved. Benzodiazepines and phenothiazines (acepromazine, Calmivet®), like most general anesthetics, do not have any known analgesic action in animals. The loss of consciousness of the animal should not be considered as a method of treating pain.
To overcome pain generated by the surgical procedure, analgesia should be determined by the intensity of pain in each individual case (Fig. 5.10). There are two main classes of analgesics: NSAIDs and morphine derivatives (opiates).
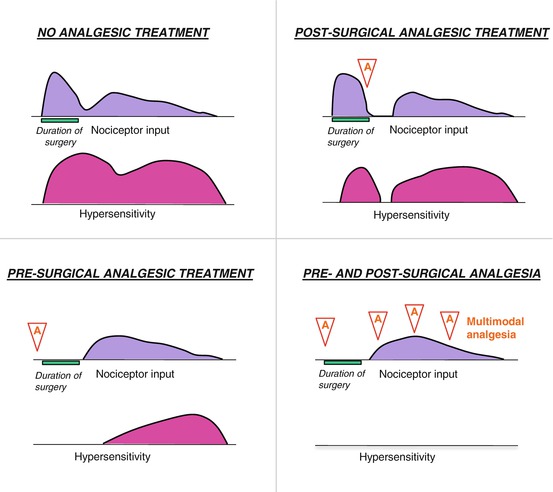

Fig. 5.10
Schematic of preemptive analgesia in preventing sensitization of the nervous system throughout the perioperative period. A analgesic drug administration. A typical experience without analgesic intervention is shown in the top left diagram, which depicts pain from the initial surgery (nociceptor input, shown in purple) and the hypersensitivity (pink) that subsequently develops. In the upper right diagram, analgesia (A) administered after sensitization may decrease pain somewhat but has little long-term benefit. Analgesia (A) administered before surgery (lower left) limits the pain from that stimulus and decreases subsequent hypersensitivity. The most effective preemptive analgesic regimen is initiated before surgery and continued throughout the postoperative period (lower right diagram) (Modified from www.aafp.org/afp/2001/0515/p1979.html)
In rodents, the use of NSAIDs, like aspirin, carprofen, ketoprofen, paracetamol, and flunixin, is often preferred. These compounds have the advantage of not causing sedation or dependence and are very effective against inflammatory pain associated with orthopedic and soft tissue surgery, infection, or trauma. In addition, there are many NSAID preparations, and there are no regulatory restrictions particular to their use or storage. In contrast, morphine and its derivatives such as buprenorphine and butorphanol oblige a much stricter and more regulated use. The choice of analgesic has implications for the larger functions of the body (Table 5.3).
Table 5.3
Principal analgesics used in veterinary surgery of rodents
Molecule | Analgesia | Antipyretic | Action on platelets and bleeding time | Gastrointestinal ulceration, hepato- and nephrotoxicity |
|---|---|---|---|---|
Carprofen (Rimadyl®) | +++ | + | NO (even chronically) | NO (even chronically) |
Paracetamol | + | ++ | NO | NO |
Aspirin | + | ++ | +++ | NO |
Flunixin | +++ | + | +++ | +++ |
Ketoprofen (Ketofen®) | +++ | + | +++ | +++ |
NSAIDs have an impact on immune function and platelet activity and may cause gastrointestinal ulceration. They also have a nephro- and hepatotoxicity that varies from one species to another. NSAIDs are also contraindicated in animals treated with anticoagulants or when coagulation or gastrointestinal functions are the object of the study’s experimental protocol.
Preferred choices are carprofen, particularly effective with a minimal risk of toxicity, and paracetamol, which has no effect on platelet activity and bleeding time, unlike aspirin. Flunixin and ketoprofen are more effective painkillers than aspirin and paracetamol but also have also greater platelet and gastrointestinal toxicities. They are thus reserved for treatment periods of short duration.
Example of an anesthetic and analgesic protocol for a rat brain implantation procedure (Fig. 5.11).
Before surgery, the animal receives an analgesic dose of carprofen (Rimadyl®).
Anesthesia is induced 15 min later by an intraperitoneal injection of xylazine and ketamine.
Localized subcutaneous injection (“infiltration”) of local anesthetics such as lidocaine or bupivacaine affords complete anesthesia at the sites of incision and the pressure points of the stereotaxic apparatus. To minimize bleeding when using lidocaine, it is advisable to use a solution of 5 mg/ml containing 2 % adrenaline (epinephrine) and diluted to ¼ in sterile water. It should not exceed 7 mg/kg, i.e., 40 μl for mouse and 350 μl for rat. The local scarring process is independent of the administration of a local anesthetic (Waite et al. 2010).
After surgery, animals receive a second dose of Rimadyl®. This treatment should be repeated once a day for at least 2 days after surgery
The veterinarian can help in establishing the anesthesia and analgesia protocol that suits each type of procedure.
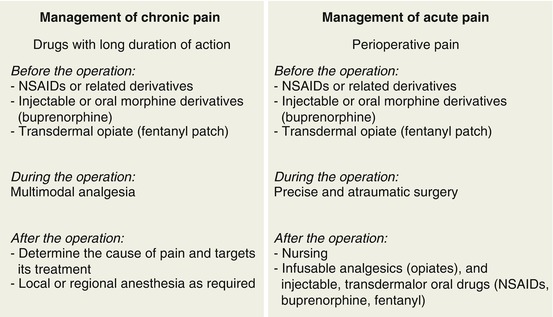

Fig. 5.11
Principal elements in the treatment of chronic or acute pain
5.3 Anesthesia
General anesthesia induces a temporary and reversible suspension of consciousness, pain sensitivity, and mobility in animals. General anesthetics are typically administered by injection or inhalation (Jensen et al. 2004). Stereotaxic procedures require a general anesthesia in order to obtain a loss of consciousness or artificial sleep, narcosis, a suppression of sensation (especially of pain), suppression of movements, muscle relaxation, as well as preservation of autonomic functions (Harrison 2003).
If the condition of the animal allows the surgery to proceed, the operator should limit stress of the animal prior to anesthesia. For example, a change in the animal’s cage environment just before surgery or bringing all of the animals into the surgery room at the same time should be avoided. Stress can be caused by various factors such as the smell of anesthetics and solvents, the movements of the operator, pheromones, and audible or ultrasonic vocalizations of the animal’s congeners.
The drugs used for anesthesia can take variable amounts of time before being metabolized and eliminated. Certain of these can persist in the body for up to 1 week after surgery. Thus, special attention should be given in choosing the type of anesthetic, depending upon being the type of experiment (electrophysiology, microdialysis, acute pharmacological treatment, etc.) that will take place after the surgery (Chery et al. submitted; Farber et al. 1997; Hemming 2009; Vizcaychipi et al. 2011).
A good anesthesia involves the combined administration of several molecules in a particular sequence. The different steps in obtaining anesthesia are defined as follows:
Premedication corresponds to the preoperative administration, 30–90 min before the operation, of one or more substances for preventing some of the effects of anesthesia, such as hypersalivation, and to reduce anxiety related to the procedure. This premedication can also allow a reduction in the dose of anesthetic by lowering the basal metabolic activity of the animal.
Induction of anesthesia is the most delicate part of anesthesia because of the complications that may arise, such as agitation, glottal spasms, hypertension, tachycardia or bradycardia, respiratory depression, apnea, atrial fibrillation, or cardiac arrest. Induction occurs immediately following administration of the anesthetic and should be as short as possible.
Anesthesia is maintained in a continuous manner to keep the animal at a surgical level throughout the procedure. Repeated reinjections or continuous infusion of anesthetic can be used to this end.
Awakening from anesthesia begins after stopping the administration of anesthetic and may be affected by the consequences of the surgical procedure.
Recovery of brain functions (motor, sensory, and cognitive) and normothermia signal the return to a normal physiological state, while the elimination of certain anesthetics may continue over several days.
The different steps in the anesthetic procedure (premedication, induction, maintenance, etc.) should not be confused with the different stages of anesthesia that define its level (depth)
5.3.1 The Different Stages of Anesthesia
The stages described below may vary in duration and intensity, depending on the anesthetic used. In addition, each species has a particular behavior during anesthesia, sometimes with marked individual variations. As anesthesia deepens, reflexes disappear in the following order:
Palpebral or blink reflex: lightly touching the eyelids causes the animal to blink. In this case, the animal is not sufficiently anesthetized.
Pinch reflex: pinching the toe or footpad of the animal with the fingers causes discomfort. If the animal withdraws its paw, it is not adequately anesthetized. Otherwise, if sufficiently anesthetized, the animal should feel no pinch or pain.
Corneal reflex: carefully touching the cornea of the eye with a tuft of cotton causes the eyelids to blink. Once the corneal reflex disappears, the animal is under excessive anesthesia.
Definition of the Different Stages of Anesthesia
Stage 1 or induction: evidenced by voluntary excitation with increased cardiorespiratory rhythm.
Stage 2 or stage of delirium and involuntary excitation: seen as a loss of progressive awareness and exaggerated responses to normal stimuli. The animal may have periodic apnea, more or less marked, and vomiting, hence the importance of fasting the animals overnight to avoid any risk of aspiration and choking. This stage should be kept as short as possible to minimize the occurrence of accidental injury to the animal.
Stage 3 or stage of general anesthesia consists of four phases, including surgical anesthesia.
Phase 1 – light anesthesia: most reflexes (palpebral, pinch, and corneal) are reduced but still present.
Phase 2 – medium anesthesia: most reflexes (blink, pinch, corneal) are absent and the muscles are relaxed. Surgeries are performed at this level; this is the stage of surgical anesthesia.
Phase 3 – deep anesthesia: pupillary reflexes (change in the diameter of the pupil in response to the intensity of light that falls on the retina of the eye) are diminished or absent. A decrease in muscle tone affects the intercostal muscles and spontaneous breathing, thus requiring assistance (artificial respiration).
Phase 4 – excessive anesthesia: complete absence of corneal reflex and paralysis of muscles, including respiratory muscles (intercostals and diaphragm).
Stage 4 – irreversible anesthesia: characterized by respiratory arrest, followed by cardiovascular collapse and death within 1–5 min.
5.3.2 Monitoring Vital Signs
Anesthesia can induce mortality and morbidity by altering the metabolism of the animal. Given their small size, rodents are quite susceptible to hypothermia and dehydration, especially during inhalational anesthesia. The following section aims to define and standardize a rigorous procedure for monitoring the experimental animal before, during, and after surgical interventions. Emphasis will be given to the principal points to consider and will not detail the multiplicity of methods and techniques that can be employed. While the priority is to monitor the proper depth of anesthesia during the operation by checking the various reflexes, attention also needs to be given to maintaining general homeostasis, body temperature, and proper hydration of the animal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








