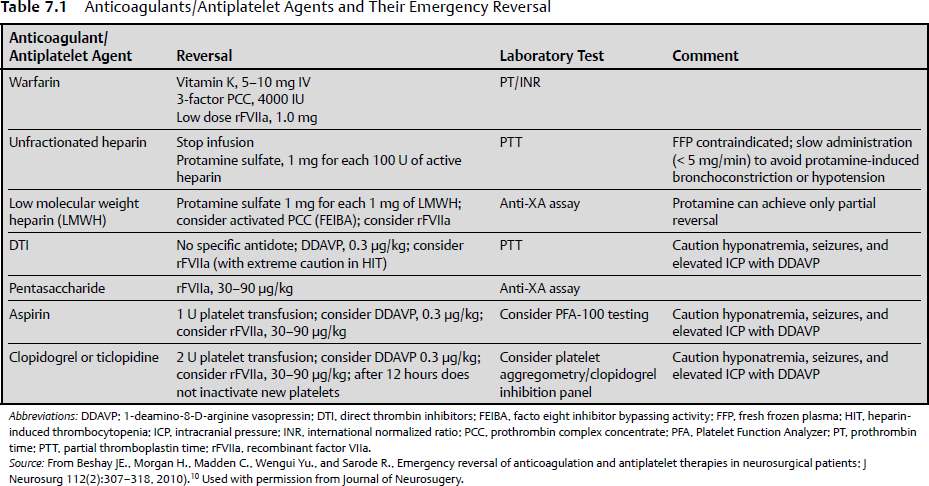
7 Blood loss, a universal concern in surgery, is only somewhat mitigated by the possibility of blood replacement by transfusion. It is better to avoid blood loss than to control or replete it. Ideally, careful surgery includes strategies and techniques to reduce blood loss, to maintain normal coagulation status, and to actively recover blood where possible. Adherence to these practices minimizes blood loss and therefore reduces its negative hemodynamic consequences and the need for transfusions. The volume of blood loss associated with a surgery highly depends on the type of surgery performed. Some neurosurgical procedures, such as clipping of a ruptured aneurysm, usually entail very little blood loss, but occasionally blood loss can be extreme. Excessive surgical bleeding causes hypovolemia, hypotension, hemodynamic instability, and anemia, and reduces oxygen delivery to tissues, with a subsequent increase in postoperative rates of morbidity and mortality.1 Steps to anticipate and reduce blood loss should be a consideration in every neurosurgical case. Allogeneic blood transfusion is associated with adverse effects, including the potential for transmission of infectious diseases, immunosuppression, transfusion-related acute lung injury, transfusion-related allergic reactions, and graft versus host reactions. The fiscal implications of blood transfusions are significant in terms of both the direct cost of a transfusion as well as costs related to additional treatments for side effects and prolonged hospitalization. At the end of the 19th century, the early evolution of the specialty of neurologic surgery was restricted by complications related to infection, increased intracranial pressure, and excessive intraoperative blood loss. These complications often resulted in mortality rates of 30 to 50%. An improved understanding of pathophysiological factors involved in increased intracranial pressure, along with meticulous surgical techniques learned from William Halsted, allowed Harvey Cushing to increase the safety of neurosurgical procedures that were then in their infancy. Cushing’s later development of the “silver clip” and incorporation of electrosurgical cautery techniques facilitated safe resection of brain tumors previously assumed to be inoperable. The inability to control bleeding from vascular lesions such as arteriovenous malformations, aneurysms, and certain brain tumors made these the last lesions to become operated on routinely. Better understanding of anatomy and fundamental measures to control blood loss, such as proximal vascular control, the use of temporary clips, bipolar electrocautery, and endovascular embolization, improved surgical outcomes. These pivotal accomplishments paved the way for the present evolution of our specialty.2 Blood makes up about 8% of human body weight and has about the same density as water. The average 70-kg male has a blood volume of about 5 L. The erythrocytic (red blood cell) component, which constitutes the hematocrit, is 40% of the blood volume in women and 45% in men. Blood components include erythrocytes, leukocytes, platelets, and plasma. All of these components are consumed or lost in the presence of bleeding, and all are important for maintaining normal health and physiologic function. Blood can be lost from arteries, capillaries, and veins. Some trauma to these structures is inevitable during the course of surgery. Nonetheless, any surgical strategy should include the goal of minimizing blood loss. A surgical route that does not violate major vascular structures and that exposes them in a controlled fashion is preferred. Anticipation of arteries during dissection, including their rapid identification and preservation or control, is a basic step. For both arteries and veins, preservation is usually preferred to sacrifice. Arterial bleeding must be controlled by electrocoagulation or ligation but should rarely be relied on to stop spontaneously. Very small arterioles may stop bleeding spontaneously, but this form of hemostasis should be regarded with healthy suspicion. When possible, the avoidance of intraoperative or early postoperative hypertension may assist such passive hemostasis. Controlled hypotension must be balanced against the essential priority that brain and spinal cord perfusion must be adequate. Capillary bleeding stops spontaneously in the presence of adequate circulating and tissue coagulation factors. Venous bleeding can often only be controlled with tamponade, frequently with preservation of the vein. Simple maneuvers such as elevation of the surgical site also may aid venous hemostasis. If, however, the site is elevated too much, so that the local venous pressure becomes negative, there is the risk of air embolism. This risk is particularly problematic in regions with noncollapsing veins (e.g., the major cranial venous sinuses).3 When possible, a patient should enter surgery with as near normal coagulation status as possible. As surgery progresses, maintenance of normocoagulation requires vigilance and anticipation. Preservation of normocoagulation is aided by normothermia during surgery, because of the deleterious effects of hypothermia on platelet function.4 Blood coagulability can also be compromised by fluid replacement as a result of hemodilution. Moderate crystalloid substitution accelerates rather than inhibits blood coagulation; however, with advanced crystalloid replacement, profound hemodilution may cause blood coagulation to become compromised.5 The use of colloids also may compromise coagulation; this effect is seen with hydroxyethyl starch more than with gelatin and serum albumin.6 The substrates of blood clotting must be sufficient to drive the clotting process. The patient’s baseline level of coagulation factors may be affected by a variety of physiological and pathological factors or conditions such as liver disease, autoimmune diseases, or von Willebrand disease, among many others. During surgery, consumption of clotting factors can lead to coagulopathy when a large volume of blood is lost or a large surface is traumatized. Disseminated intravascular coagulation can also consume necessary factors. Finally, extensive brain tissue trauma (e.g., with gunshot wounds to the head) can release tissue thromboplastins, resulting in ineffective coagulation. Surgical control of bleeding usually involves gross closure of bleeding vessels with electrocautery, clips, or ligatures. Electrocautery coagulates proteins in blood vessel walls and surrounding tissues, occluding the lumen. This kind of closure is fairly reliable as long as generous closure of the vessel is achieved. As a rule, arteries require more coagulation than veins. Tumor vessels or arteriovenous malformation vessels sometimes require more coagulation with cautery than normal vessels. Larger vessels (greater than 1–2 mm in diameter) are often best closed with a clip. Temporary and permanent aneurysm and arteriovenous malformation clips that are rated to closing pressures that exceed even the highest possible supranormal physiological pressures are commercially available. Titanium or other alloys that are compatible with magnetic resonance imaging (MRI) should be used when possible. Proximal control of bleeding with clips must proceed with caution, because the need to control blood loss must be balanced against the risk of ischemia in the distal territory of the involved vessel. The following sections discuss the physiology of hemostasis, pharmacological and topical hemostatic agents for controlling blood loss, the emergency reversal of anticoagulation and antiplatelet therapies in neurosurgical patients, and control of blood loss in specific neurosurgical situations. In response to blood loss, the body compensates physiologically to maintain blood pressure and perfusion of key organs. At first, tachycardia occurs to increase cardiac output. Concomitantly, vasoconstriction occurs peripherally, supporting the blood pressure by decreasing flow to the skin and muscle. Vasoconstriction proceeds to shift flow away from other nonessential organs, such as the gastrointestinal tract and kidneys, while maintaining flow to the brain, heart, and lungs. As acidosis and hypercarbia occur, a shift occurs in the hemoglobin-binding curve to promote release of oxygen. The impact of blood loss is a function of both the volume and rate of blood loss. There is no good formal classification of neurosurgical blood loss or even of surgical blood loss in general. Blood loss related to hemorrhage, such as that sustained in trauma, is divided into four classes by the American College of Surgeons in its Advanced Trauma Life Support (ATLS) courses.7 Although surgical bleeding may follow a slightly different course because it is actively managed and occurs under anesthesia, the general classification is useful. A class I hemorrhage constitutes blood loss of 15% or less of circulating blood volume. Hemodynamic changes in the vital signs are seldom related to physiological compensation such as mild vasoconstriction, which is often not clinically apparent. Class II hemorrhage involves loss of 15 to 30% of blood volume. Tachycardia is necessary to compensate for the loss of volume by increasing cardiac output. The pulse pressure may narrow, and clinically apparent vasoconstriction, such as cooling of the extremities and blanching of the skin, may occur. Fluid resuscitation with crystalloids may be adequate to reverse this response. Class III hemorrhage is defined as a loss of 30 to 40% of circulating blood volume. With this degree of blood loss, tachycardia increases, the blood pressure falls, and more intense peripheral vasoconstriction occurs. Compensation occurs in the periphery and in some solid organs that would be unsustainable if the blood loss is not eventually reversed. Fluid resuscitation with volume expanders will be necessary. If available, blood transfusion is considered at this point. Class IV hemorrhage is defined as a loss of 40% or more of circulating blood volume. Physiological mechanisms cannot compensate for the blood loss. If aggressive resuscitation with volume replacement, volume expanders, or blood is not instituted, death occurs. Healthy patients may be able to compensate more fully for blood loss because of a better ability to increase heart rate, stroke volume, or vascular tone. Older or less healthy patients may suffer the ill effects at lower degrees of blood loss, or they may suffer permanent damage at an earlier stage despite a lesser degree of blood loss. These numbers also can serve as a rough guide during surgery. Typically, blood loss on the order of 15% or less is well tolerated during surgery, and no replacement other than crystalloid or colloidal fluids is necessary. As blood loss increases, resuscitation should be ongoing and should escalate through colloids to blood products. Careful anesthesia monitoring for signs of cardiovascular instability, hypotension, and possibly cardiovascular collapse is essential. If only colloids are used, the hematocrit and hemoglobin concentration begins to fall through hemodilution. However, there is no strict hematocrit percentage that is considered critical for blood replacement. This level depends on several patient factors. For blood loss to be minimized during neurosurgical procedures, the patient should have a functioning hemostatic system. Hemostasis depends on a successful balance among the coagulation, complement, and fibrinolytic pathways. Complex interactions are necessary between plasma proteins, platelets, blood flow and viscosity, and the endothelium. Spontaneous clotting involves formation of the primary hemostatic plug at the site of a damaged vessel wall, which is the first event in the control of bleeding. In this mechanism, the glycoprotein (GP) Ib receptor on platelet membranes binds to von Willebrand factor (vWF), which is linked to the exposed subendothelium. This process enables platelets to adhere to the site of injury. Adherent platelets become activated and undergo a conformational change, increasing their surface area contact with the subendothelium. Activated platelets also release adenosine diphosphate (ADP) and thromboxane A2, which, in concert with thrombin derived from the coagulation cascade, stimulate platelet aggregation via receptor-mediated metabolic processes. ADP, thromboxane A2, and thrombin bind their respective platelet membrane receptors, thereby activating the platelet surface receptor GPIIb/IIIa to bind soluble extracellular ligands such as plasma fibrinogen and vWF. These ligands link to GPIIb/IIIa receptors on adjacent platelet membranes, enhancing the initial adhesion and allowing other platelets to accrete on those already attached, thus forming a platelet plug at the site of injury.8 Coagulation involves the laying down of a strong fibrin mesh through the primary platelet plug and is brought about by the action of a sequence of proenzymes and cofactors that work in concert to generate the final enzyme, thrombin. Thrombin then directly cleaves fibrinogen to produce fibrin. The enzymes involved in blood coagulation belong to the serine protease family. This class of enzymes has a common mechanism of action that requires the catalytic triad of serine, aspartic acid, and histidine within the active site. Activation of the coagulation cascade triggers several physiological pathways that tend to counteract and limit the spread of coagulation to the area of injury. This balance is necessary to allow targeted clotting that prevents further blood loss but does not interfere with flow in normal vessels. As patients are increasingly treated with anticoagulants and antiplatelet agents, it is essential that physicians who care for them understand the pathways these drugs act on and the degree to which they can affect perioperative blood loss, and that they recognize how these drugs can be manipulated to restore hemostasis9 (Table 7.1). Not only does the risk and incidence (7- to 10-fold greater) of intracranial hemorrhage increase in patients on anticoagulants compared with those who are not, but anticoagulant-induced hematomas tend to be larger and more likely to expand.10–13 Clopidogrel (trade name Plavix) and ticlopidine (Ticlid) are drugs that inhibit the P2Y12 ADP receptor of platelet membranes, preventing activation of the GPIIb/IIIa pathway and thus platelet aggregation and clot formation. Because there is a finite amount of circulating metabolite available to inactivate existing platelets, the anticoagulant effect of these drugs can be corrected with platelet administration. Another frequently used anticoagulant, Coumadin, acts by depleting vitamin K–dependent coagulation factors (factors II, VII, IX, and X, protein C, and protein S). Correction of the international normalized ratio (see Chapter 9) involves replacement of vitamin K and the dependent coagulation factors. A few options are available for replacing coagulation factors: fresh frozen plasma, prothrombin complex concentrate (PCC), and factor VIIa. PCC is available in a three- or four-factor form. The four-factor form includes factors II, VII, IX, and X, whereas the three-factor form includes factors II, IX, and X only and must be supplemented with additional factor VII. Because transfused factors have a limited half-life, vitamin K must always be administered for further hepatic production and replacement of factors.9 Dabigatran etexilate is the first oral direct thrombin inhibitor available for anticoagulation. It presents a challenge, because the typical reversal agents (fresh frozen plasma, PCC, and vitamin K) do not reverse its anticoagulant effects, and it is not easily monitored.14 A limited number of pharmacological agents have been used to correct hemostatic defects in the perioperative period. The lack of clear understanding regarding the pathogenesis of perioperative bleeding as well as its multifactorial origins has limited the development of specific and new hemostatic agents. Although several good agents are available to assist surgeons with the intraoperative control of bleeding, highly specific agents for the control of neurosurgical bleeding do not exist. One agent that has been used intraoperatively is desmopressin. Two studies have evaluated the use of desmopressin in patients undergoing spinal fusion surgery. Kobrinsky and colleagues15 reported a beneficial effect in terms of blood loss and transfusion requirements, whereas Guay and colleagues16 found no benefit.
Principles of Blood Loss
History
Blood Volume and Constituents
Factors Affecting Blood Loss
Physiological Impact of Blood Loss
Physiology of Hemostasis
Coagulation Cascade
Emergency Reversal of Anticoagulation and Antiplatelet Therapies
Pharmacological Hemostatic Agents
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree