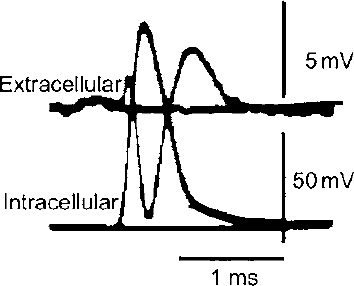
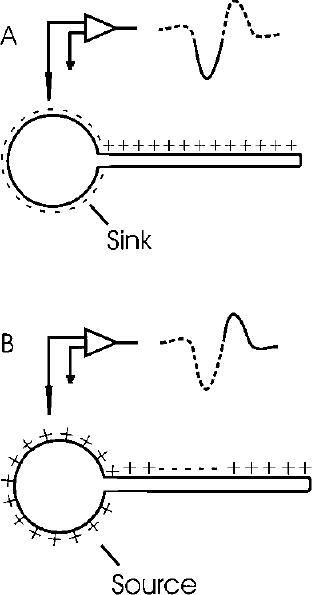
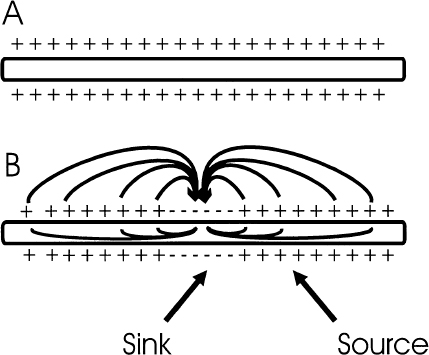
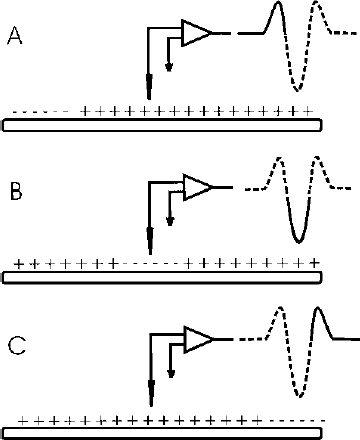
2 There is a rather large gap between membrane properties of individual neurons and behavior or physiological events. To bridge this gap, we need to be able to understand the activity of individual neurons and how that activity contributes to neuronal circuits. Extracellular microelectrode recording, which focuses on neuronal action potentials of individual neurons as the “code” used by the intact nervous system for transmitting information, is the method of choice for investigating questions at this level of analysis. Because the extracellular recording method allows an investigator to record the discharges of a single neuron without impalement, a single cell can be isolated and studied for hours, or even days, in chronic recording studies in animals. Individual neurons can thus be characterized comprehensively in terms of physiology and connectivity, and cell activity can be correlated with behaviors or physiological events. Electrical or chemical stimulation of identified cell populations at the recording site can also be used to determine the effects of cell activity on behavior or physiology. As with any methodology, there are limits to the kinds of questions that can be addressed with extracellular microelectrode recording. At the membrane level, subthreshold events, such as synaptic potentials that might influence cell excitability without producing an action potential, remain hidden. Recording subthreshold potentials requires an intracellular or membrane level approach. Investigators focusing on subthreshold events for the most part have moved to in vitro preparations where intracellular and patch recording techniques are much more easily applied than in the whole animal. At the level of neural circuits, an extracellular electrode samples activity of only one or relatively small numbers of neurons. It can therefore be difficult to develop a realistic picture of the functioning of a large neuronal ensemble or circuit with this approach. Multiunit or slow potential recording and imaging techniques can often be more informative for analyses of distributed circuits. Nevertheless, whether the scientific interest is in events at the cell membrane or in circuits distributed across broad regions of the central nervous system, interpretation of the data will be enhanced by information about the physiology of the relevant neurons functioning within the intact nervous system. Extracellular microelectrode recording can provide this information with high spatial and temporal resolution. For the purposes of extracellular microelectrode recording, our interest is in the action potential as the code used by the neuron for information transmission. The task is thus to detect whether or not an action potential has occurred at a specific time; the waveform itself can be considered to carry no information (other than to serve for spike identification; see below). Indeed, the actual waveform is usually discarded after spike sorting has been completed. It is nonetheless of some interest to consider the basis for the action potential obtained with an extracellular microelectrode. The action potential or “spike” recorded with an extracellular microelectrode is produced by currents that are induced to flow in the extracellular space around an active neuron. A straightforward approach to these current flows is provided by volume conductor theory.1,2 In this approach, the neuron is visualized as surrounded by an extracellular medium with low uniform resistance; that is, a “volume conductor.” In the simplest case, one can imagine an isolated axon in a saline bath. When the axon is at rest, the membrane potential is uniform along its entire length, and there is no current flowing inside or outside the cell (Fig. 2–1A). However, if the axon is depolarized at some point along the membrane, the potential difference between the depolarized and resting regions will cause current to flow (Fig. 2–1B). Current flows inward at the active region, and an electrode that is adjacent to the axonal membrane at this point will be negative with respect to a distant indifferent electrode. The active region is referred to as a current “sink.” Inactive regions of the membrane adjacent to the depolarized area are then said to act as a “source” of current for the active region. Because current flows outward at the source, an electrode recording from a region of membrane that is acting as a current source will be positive relative to a distant indifferent electrode. FIGURE 2–1 Volume conductor theory can be used to model current flows around an axon in a uniform, low-resistance bath (a “volume conductor”). (A). When the axon is at rest, the membrane potential is uniform, and no current flows. (B). Current will flow when a segment of the membrane is depolarized. The flow is inward at the depolarized region (“sink”) and outward at adjacent regions, which act as a “source” of current for the sink. At least in theory, one should be able to model the distribution of sources and sinks associated with action potential discharge in an active neuron. This proves to be a straightforward task when considering the isolated axon (Fig. 2–2). As an action potential approaches, reaches, and then moves away from an electrode adjacent to a spot somewhere along the axon, the electrode will at first register a positive potential as the membrane under the electrode serves as a current source for the depolarized membrane some distance away. Then, when the action potential reaches the region underlying the electrode, the depolarized membrane will act as a sink. The extracellular electrode will therefore record a negative potential. Finally, as the action potential continues past the electrode, the underlying membrane is repolarized and once again serves as a current source. The recording electrode will therefore again be positive relative to a distant reference electrode. The action potential recorded by an electrode adjacent to an isolated axon should therefore be triphasic. This theoretical prediction has been confirmed, as shown in the example in Figure 2–3, which is from an early experiment by Terzuolo and Araki3 in 1961. They used a double electrode for simultaneous recording of intracellular and extracellular potentials during antidromic activation of a motoneuron in the ventral horn of the cat. As predicted by volume conductor theory, the potential recorded with the extracellular electrode was triphasic, and the negative phase of the extracellular spike coincided with the depolarization seen by the intracellular electrode. The late positive phase of the extracellular potential corresponded to the repolarization of the membrane recorded intracellularly. FIGURE 2–2 Model of sources and sinks predicts that a triphasic waveform will be recorded from an isolated axon. (A). As the action potential approaches the region underneath the electrode, that membrane serves as a source, and the electrode sees a positive potential relative to a distant indifferent electrode. (B). When the action potential reaches the membrane underlying the membrane, the electrode records a negative potential. (C). As the action potential continues down the axon, the membrane under the electrode once again acts as a source, and as a consequence, the electrode records a positive potential. FIGURE 2–3 Simultaneous recording of the intracellular and extracellular spike with a double electrode. The monophasic depolarization seen by the intracellular electrode corresponds with a positive-negative-positive waveform recorded by the extracellular electrode. Note different scales for extracellular and intracellular recordings. (With permission from Terzuolo CA, Araki T. An analysis of intra- versus extracellular potential changes associated with activitiy of single spinal motoneurons. Ann NY Acad Sci. 1961;94:547–588.) Similar considerations can be applied when modeling the action potential recorded close to the cell soma. As shown in the simple neuron model in Figure 2–4, depolarization at the soma should be reflected in negative potential at the electrode. The soma membrane would subsequently become a source as the action potential moved away down the axon. As a result, the waveform recorded with an extracellular electrode near the soma should in theory be biphasic, with an initial negative component followed by a later positivity. This general pattern has been confirmed for at least some neurons in the central nervous system (Fig. 2–5).3–5 However, actual neurons are somewhat more complex than the “lollipop” envisioned in Figure 2–4. Cell morphology and distribution of active conductances on the somatic and dendritic membrane, the location of the electrode relative to the cell body, and the state of cell excitability all complicate the simple picture derived from volume conductor theory. As a result, somatic recordings vary in the relative amplitude of the negative and positive phases of the waveform and often show distinct inflection points on either phase.
Principles of Extracellular Single-Unit Recording
MARY M. HEINRICHER
Extracellular Recording: Neuronal Activity in a Functional Context
What Is an Extracellular “Spike”?
Neupsy Key
Fastest Neupsy Insight Engine