ProdiscC™ Cervical Disc Prosthesisin the First 68 Patients: One-Year Evaluation of Results
Petr Suchomel
Pavel Barsa
P. Sourkova
P. Buchvald
R. Fröehlich
J. Hradil
A promising experience with lumbar disc functional replacement led investigators to the development of mobile disc prostheses for the cervical spine. The search for dynamic devices was initiated following reports by Hilibrand and Carlson (8) and Goffin and van Loon (5), describing adjacent segment overload in the long term after cervical fusion procedures. Overall results of anterior cervical discectomy and fusion (ACDF) are very good (3,11,16,19); however, nowadays there is also a desire to preserve at least a part of a spine segment motion, simply to adapt our surgical efforts to the natural spine behavior.
Initial clinical success of the first-generation (6,18), simplified introduction technique and advanced design of the second generation of cervical prostheses has changed the indication philosophy in the treatment of cervical degenerative disc disease (cDDD), resulting in increased frequency of implantations (2,4,9,14). We present our experience with the first 68 patients treated with ProdiscC™ prosthesis and include a detailed analysis of 1-year follow-up data in 30 of them.
Material and Methods
Between February 2004 and August 2005, we performed anterior cervical discectomy in 170 patients with cDDD. Seventy-nine ProdiscC™ mobile disc prostheses were used in 68 patients; single level in 58 patients, two levels in 9 patients, and three levels in 1 patient.
Indication for surgery was based on harmony of clinical and radiologic findings. Most of our patients suffered from radiculopathy and/or myelopathy. Purely axial pain was an indication only in three cases. Soft disc herniation with or without spondylosis were the main reasons for total disc replacement in our series. Well-preserved motion in the target segment on dynamic radiographs and disc height greater than 3 mm preoperatively were the main indication criteria in the decision-making process. Conversely, mechanical segmental instability as well as patient’s age younger than 20 years or older than 65 years were the contraindications to the procedure. Other contraindications were similar to those for cage implants (osteoporosis, allergy, etc.).
At the time of this publication, 30 patients (39 implants) were available for 1-year follow-up. This group is further analyzed in detail.
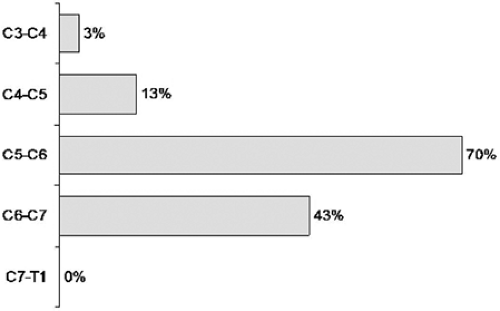
Average age of these patients was 45 years (range 21–60 years). Male gender was slightly more prevalent—57%. Most of our patients (60%) were overweight or obese according to body mass index measurements. Preoperatively 60% of them were fully employed, 7% in part-time employment, and 33% out of work due to their cervical disc disease. All, except those with rapid progression of neurologic deficit, were treated conservatively for at least 8 weeks preoperatively. The most frequently involved segments were the C5-6 and C6-7 levels (Fig. 28.1). The majority of patients (22 patients) required only a single-level operation. A two-level procedure was performed in 7 and three-level in 1 patient in this group.
Surgery
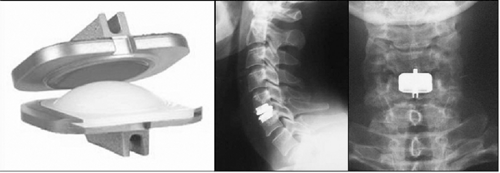
A standard, right-sided anterolateral approach was performed in all cases. After a total anterior discectomy, posterior osteophytes were removed when necessary. The posterior longitudinal ligament was resected in the majority of cases. Trial implant was used to determine the proper height and size of implant. Primary stability of ProdiscC™ implant is achieved by anchoring of central keels in bone of the endplates (Fig. 28.2). The keel grooves were cut with specialized chisel guided by the trial implant. Finally, the implant was simply introduced attached to the implant holder similarly to cage application. All steps of the disc implantation were monitored with the aid of lateral fluoroscopy.
External semirigid collar was used only for the duration of the recovery from general anesthesia. All patients were mobilized immediately thereafter.
Outcome Measures
Clinical, Neck Disability Index (NDI), visual analogue scale (VAS), and radiologic follow-up data were collected immediately after surgery, at discharge, 6 weeks, 6 months, and 1 year after the operation. Neurologic status and VAS scoring was evaluated by an independent neurologist, and radiologic results were reviewed by two involved surgeons (S.P. and B.P.). NDI data were collected by an independent party over the phone. All patients included in this prospective long-term study signed an informed consent.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree