Fig. 6.1
The anatomical connections evaluated by the median nerve SSEP
SSEPs are usually evoked by bipolar transcutaneous electrical stimulation applied on the skin over the selected nerve and registered with disc electrodes along the tract. For example, in recordings of the median nerve, registration electrodes can be placed at the elbow, Erb’s point (over the plexus, above the clavicle) and cervical, parietal and frontal cortex (Fig. 6.2; see also Chap. 2). Cortical responses can only be interpreted reliably when the peripheral responses are present. In the nomenclature of SSEP waveforms, N or P followed by an integer is used to indicate the polarity (positive, respectively, negative) and the nominal poststimulus latency (in ms) of the recorded wave in a healthy reference population (e.g. P15, N20). The earliest cortical potential is the N20, which is generated in the primary somatosensory cortex, where thalamocortical cells undergo synaptic connections with the superficial and deep pyramidal cell layers (Allison et al. 1991). In comparison to cortical responses of greater latency, the N20 is the most robust, as this is the latest waveform to disappear following increasing levels of encephalopathy or pharmacological sedation; of note, however, the N20 is relatively independent to the level of sedation used in clinical settings (Cruccu et al. 2008). Since the cortical waveforms appearing later (such as P45, N60 and P/N100) are less reliable and more susceptible to changes by sedation, the N20 is widely used in almost all clinical prognostic questions.
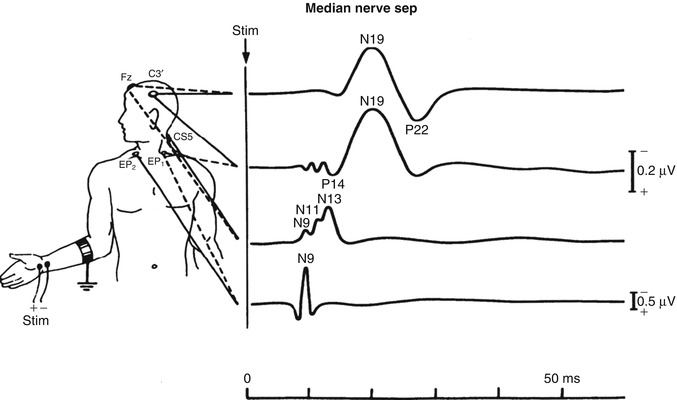

Fig. 6.2
Multiple recording sites of SSEP
6.3 Pitfalls and Limitations of SSEP Recordings in the ICU
One of the main problems of the SSEP interpretation is the interobserver agreement, which has been extensively described in several studies (Zandbergen et al. 2006a; Pfeifer et al. 2013). Zandbergen et al. investigated 56 consecutive patients with anoxic–ischaemic coma (Zandbergen et al. 2006a); these registrations were interpreted independently by five experienced clinical neurophysiologists. The interobserver agreement for SSEPs in anoxic–ischaemic coma was only moderate (kappa 0.52, 95 % CI 0.20–0.65): the main source of disagreement was related to the underlying electrical noise, implying difficulties in obtaining a reasonable signal-to-noise ratio. For recordings with a noise level of 0.25 μV or more, the mean kappa was as low as 0.34 (fair agreement), while for recordings with noise levels below 0.25 μV, the mean kappa improved to 0.74, which is a substantial agreement. Similar results have been reported by Pfeifer et al., again in subjects admitted after cardiac arrest (Pfeifer et al. 2013). One way of integrating the SSEP information with other neurophysiological variables, in order to limit the aforementioned problems, may be a continuous SSEP registration combined with continuous EEG. This can be used to monitor deterioration in patients with severe brain injury (Amantini et al. 2009); however, such approaches are still rare in clinical practice. Of note, almost all literature regarding the use of SSEP for prognostication uses the absence or presence of short-latency cortical responses (N20). Whether the amplitude of cortical responses can be used is uncertain. During continuous SSEP and EEG registration, an N20 amplitude <1.2 μV has been used as a cut-off to describe an abnormal SSEP (Bosco et al. 2011), but it is important to underscore that this threshold does not reflect any evidence nor current practice for N20 analyses in the prognosis after cardiac arrest.
Efforts should be made to increase the signal-to-noise ratio as much as possible. As an orienting threshold, Zandbergen et al. recommend that the peak-to-peak amplitude of noise of the cortical and cervical leads should be lower than 0.25 μV after averaging, especially in the frequency of the SSEPs themselves (20–500 Hz) (Zandbergen et al. 2006a). Administration of muscle relaxants (together with modest doses of sedation, such as 5 mg of midazolam) often improves the quality of the SSEP registrations in patients with abundant muscle activity. Furthermore, disturbing electrical ICU equipment should be turned off as much as possible. Also, delivering more stimuli (up to 1,000 or more) and increasing the stimulus intensity could contribute to optimize the signal-to-noise ratio. In addition, it has been also suggested that the stimulus rate may influence the results of an SSEP recording (Robinson and Micklesen 2010) (see Chap. 2). Since the interpreting clinician is often not present during the actual SSEP registration itself, the role of the technician is crucial in obtaining reliable results.
6.4 Interpretation Criteria of SSEP
Additional criteria apart from the signal-to-noise ratio should be kept in mind when interpreting SSEP recordings. An N20 peak on one side can only be considered as present if it fulfils all of the following criteria (Zandbergen et al. 2006a):
It should have an appropriate latency (i.e. appearing at least 4.5 ms later than the corresponding N13 peak recorded from the posterior cervical region in normal-stature adults).
It should be present on the other side, and there should be a clear difference with the recording from the side ipsilateral to the stimulus. Therefore, it is recommended to record not only the contralateral sensory cortex after stimulation but also co-register the ipsilateral side. This prevents misinterpretation of the N18, which has its origin in the brainstem, as an N20 potential.
Any potential should be clearly reproducible in a second set of stimuli.
Bilateral absence of N20 peaks requires the presence of normal potentials over Erb’s point and the neck (N13), in order to ensure that the impulses have arrived in the central nervous system through an integer peripheral pathway. Figure 6.3 illustrates an example of an SSEP registration with present and absent N20 cortical responses.
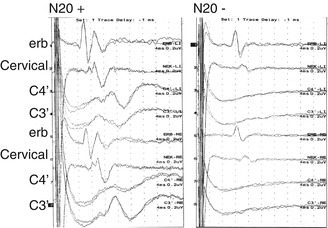

Fig. 6.3
Median nerve SSEP registration with and without cortical responses
6.5 Confounding Factors and Sedation
Cortical responses are generally not influenced by moderate pharmacological sedation or metabolic disturbances, factors that often hamper the clinical neurological examination in the ICU. However, massive intoxications, very severe biochemical or metabolic disturbances and anatomical (e.g. a high cervical) lesions should be actively ruled out. The cortical N20 responses may remain still visible even at sedation levels sufficient to induce an isoelectric EEG (Cruccu et al. 2008; Rothstein 2004); nevertheless, care should be taken when high-dosed barbiturates are administered: high-dosed sodium thiopental induces an increase in latencies and decrease in amplitudes for median nerve SSEP and brainstem auditory evoked responses (Drummond et al. 1985). It is uncertain whether the amplitudes decrease to a level that the cortical response can no longer be identified. Patients with absent cortical responses during thiopental (or pentobarbital) coma prescribed to treat increased intracranial pressures who made a good recovery in the end have been reported in the literature (Robe et al. 2003). This suggests that very high-dosed barbiturates can depress SSEP cortical responses. Propofol produces minimal suppression of the SSEP amplitude, which at most may be quantified to a loss of less than 10 % (Langeron et al. 1999). Also, midazolam and opioids have only moderate effects on SSEP amplitudes and latencies (Langeron et al. 1999; Asouhidou et al. 2010; Laureau et al. 1999; Taniguchi et al. 1992). Remifentanil can lower the cortical components by 20–80 % when given at a high dose (0.8 mcg/kg/min) as used during neuromonitoring in the operating room (Asouhidou et al. 2010). On the other hand, as stated above, in some cases it may be even advisable to administer low-dose sedation to improve the quality of the SSEP recordings. This is especially the case in patients with generalized periodic discharges on the EEG (see Chaps. 3, 4 and 5), which in some situations can be suppressed after the administration of propofol. These periodic discharges often have larger amplitudes in comparison to the evoked potentials and can disturb the detection of cortical response.
6.6 Prognostication in Postanoxic Coma
Bilateral absence of short-latency (N20) SSEP responses has been identified as the most powerful prognosticator of poor outcome in patients who remain unconscious after a circulatory arrest (Rossetti et al. 2010; Bouwes et al. 2012). In patients not treated with hypothermia, bilateral absence of cortical N20 responses 24 h or more after the event represents a reliable predictor for a poor neurological outcome (which is understood as no recovery of awareness) (Zandbergen et al. 2006b). A recent systematic review of all SSEP registrations reported in patients admitted to the ICU after resuscitation from a cardiac arrest and treated with hypothermia showed a false-positive rate (FPR) as low as 0.007, with a 95 % confidence interval of 0.001–0.047 (Kamps et al. 2013). These registrations were performed after return of normothermia. Even registration during therapeutic hypothermia might already have a solid good prognostic value, but the confidence interval is wider (Tiainen et al. 2005; Bouwes et al. 2009).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







