Comment
Grade
Appearance
Normal
I
Predominant α with rare θ
Mildly abnormal
II
Predominant θ with rare δ
Moderately abnormal
III
Predominant δ
Severely abnormal
IV
Predominant δ with brief isoelectric intervals
Extremely abnormal
V
Nearly flat or flat record
Based on his personal experience, in 1988 Synek refined the prognostic classification for comatose patients after traumatic injury or cerebral anoxia, adding complementary information to the five original grades and introducing some specific EEG patterns, in an attempt to allow a classification of as many traces as possible (Synek 1988). He also made the important observation that the prognostic significance of EEG should be assessed not too early (i.e., within a few hours) after the beginning of coma (Synek 1988). The breakdown into several categories renders the classification very accurate on the one side, but also somewhat unpractical (Table 5.2); the Hockaday grades are scattered among different prognoses, as is the background reactivity. Ten years later, Young and colleagues proposed an updated system based on their observation of 92 comatose patients, mostly postanoxic, some with traumatic injury (Table 5.3), and compared it to the Synek classification, finding a higher interobserver agreement (this was, however, tested among just two interpreters) and underscoring again the relevance of EEG reactivity (Young et al. 1997). Furthermore, they pointed out that burst suppression implies flattening for at least 1 s/20 s, while Synek did not specify the denominator.
Table 5.2
The Synek prognostic classification of EEG changes in postanoxic and brain trauma patients
Comment | Grade | Appearance |
|---|---|---|
Optimal | I | Predominant α with rare θ |
Benign | II | Predominant θ, reactive |
III | Spindle pattern | |
III | Frontal rhythmic δ | |
Uncertain | II | Predominant θ, not reactive |
III | Diffuse δ (regardless of reactivity) | |
III | Diffuse δ with epileptiform discharges | |
IV | α pattern coma, reactive | |
Malignant | III | Low amplitude δ |
IV | Burst suppression | |
IV | Burst suppression with epileptiform discharges | |
IV | α pattern coma, not reactive | |
IV | θ pattern coma | |
Fatal | IV | Low-output EEG (<20 μV δ activity) |
V | Isoelectric EEG |
Table 5.3
The Young prognostic classification of EEG changes in postanoxic and brain trauma patients
Category | Subcategory |
|---|---|
I: θ/δ >50 % of the record | Reactive |
Not reactive | |
II: Triphasic waves | |
III: Burst suppression | With epileptiform activity |
Without epileptiform activity | |
IV: α/θ/spindle coma (unreactive) | |
V: Epileptiform activity(not in burst suppression) | Generalized |
Focal | |
VI: Suppression | Between 10 and 20 μV |
≤10 μV |
This illustrates the need for more uniformity in order to allow a generalizable understanding of what is described on EEG recordings. Very recently, a common effort of several North American experts has produced a detailed description of the EEG terminology in an intensive care setting (Hirsch et al. 2013). While unequivocal electrographic seizures should show generalized spike-wave discharges >3 Hz, or clearly evolving discharges of any type reaching a >4 Hz frequency, other recurrent patterns (periodic or rhythmic, but which would not be necessarily labeled as seizures) represent the subject of this classification, intended primarily to match a research need. The first main term is chosen according to the spatial distribution (i.e., generalized, lateralized, bilateral independent, or multifocal) and the second to describe the type of transients (i.e., periodic discharges, rhythmic delta activity, or spike–waves); to qualify, the discharges should recur at least six times. Then, modifiers come to play, such as prevalence over the recording, duration, frequency, sharpness, amplitude, and stimulus induction. The EEG background is described according to symmetry, predominant posterior frequency, reactivity, voltage, sleep transients, and continuity (i.e., suppression implies the whole recording being <10 μV, burst suppression that 50–99 % of the recording is attenuated, while a discontinuous trace is attenuated over 10–50 %). A comparison of this classification with historically common terms is given in Table 5.4; the aim to disentangle the descriptive terminology of recurrent discharges from an “epileptiform” connotation is clearly recognizable. While this very precise approach represents a seminal step in the optimal direction and appears very appropriate to serve as a basis for clinical EEG research in the intensive care environment, it may again be too detailed to allow its routine use in clinical practice, and it is just beginning to undergo validation in different geographical and clinical settings.
Table 5.4
Older EEG terms and the newer terms after the standardized critical care EEG terminology proposed by the American Clinical Neurophysiology Society
Older terms | Newer terms |
|---|---|
PLEDs (periodic lateralized epileptiform discharges) | LPDs (lateralized periodic discharges) |
PLEDs+ | LPDs plus (fast, rhythmic) |
BIPLEDs (bilateral independent periodic lateralized epileptiform discharges) | BIPDs (bilateral independent periodic discharges) |
GPEDs (generalized periodic epileptiform discharges) | GPDs (generalized periodic discharges) |
Triphasic waves, most of the record | GPDs with triphasic morphology |
FIRDA (frontal intermittent rhythmic delta activity) | GRDA (generalized rhythmic delta activity, frontal predominant) |
SIRPIDs (stimulus-induced rhythmic, periodic, or ictal discharges) | SI-GPDs or -RDA or -SW (spike-waves) |
Lateralized seizure, δ frequency | Evolving RDA |
5.2 Particular EEG Patterns Found in Patients with Consciousness Impairment (See Also Chaps. 2 and 3)
5.2.1 Background Slowing and Reactivity
Several decades ago it has been observed, in cats, that lesions confined to the cerebral cortex lead to attenuation of the alpha background, while subcortical lesions deafferenting the cortex induce polymorphic delta slowing (Gloor et al. 1977); unsurprisingly, this seems to apply also to humans (Kaplan and Rossetti 2011). The possible etiologies are extremely broad, including traumatic, infectious, ischemic, or hemorrhagic causes; importantly, a “lesion” should not only be understood as a structural defect but (especially for the cortex) may also encompass functional disturbances, such as alteration of the physiological blood flow (either locally or systemically, e.g., during a migraine attack or a syncope), metabolic disturbances, or the effects of sedative medications.
The previous section and Tables 5.1, 5.2, and 5.3 illustrate well the prognostic correlation of an increasing background slowing; it is however paramount to always perform activation procedures to test the background reactivity, including alerting sounds (or calling the patient’s first name), eye opening, and painful stimulations. As a practical rule of thumb, to prevent biases owing to peripheral nerve impairment, it seems reasonable to apply the stimuli on the face or the trunk; furthermore, in order to allow the EEG to resume its non-stimulated background, stimulations should be performed at least 20–30 s apart. Even if a given recording appears very slow, a clear reactivity, understood as a reproducible change in terms of frequency and amplitude (regardless of the fact that either acceleration with amplitude attenuation or high-voltage slowing appears), heralds a relatively better prognosis (Markand 1984; Rossetti et al. 2010, 2012; Synek 1988; Young et al. 1997). Furthermore, in the era of portable video EEG, the correlation of stimuli application with the EEG signal is now very easy to assess.
5.2.2 Triphasic Waves
These EEG transients have been known for many decades and owe their appearance in the EEG literature to their seminal observation in patients with hepatic impairment (Foley et al. 1950). Triphasic waves are described as sharp deflections with two or three phases, where the second one has the highest amplitude and is generally surface positive; at times, a phase lag (i.e., a slight delay of a few tens of milliseconds of the positive wave in the anteroposterior or posteroanterior respect) may be observed (Fig. 5.1). These transients that often can be transitorily attenuated along with variation in consciousness are by no means specific to liver disturbance, as they have been observed with other metabolic disorders, as well as infections, neoplastic or ischemic lesions (Sutter et al. 2013b), intoxications, and prion diseases. They should be considered possibly epileptiform if occurring strictly unilaterally (Pohlmann-Eden et al. 1996); in this case they usually do not show any clear reactivity. While, again, reactivity has been described as favorable in terms of outcome (Sutter et al. 2013b), it is their evolution over time, rather than their mere presence, that should orient on prognosis: triphasics tend to disappear relatively quickly following correction of metabolic disorders, while they worsen in neurodegenerative conditions. Of relevance, triphasic waves may be attenuated or abolished by benzodiazepines; their disappearance in this context is thus by no means specific for an epileptiform nature (Fountain and Waldman 2001).
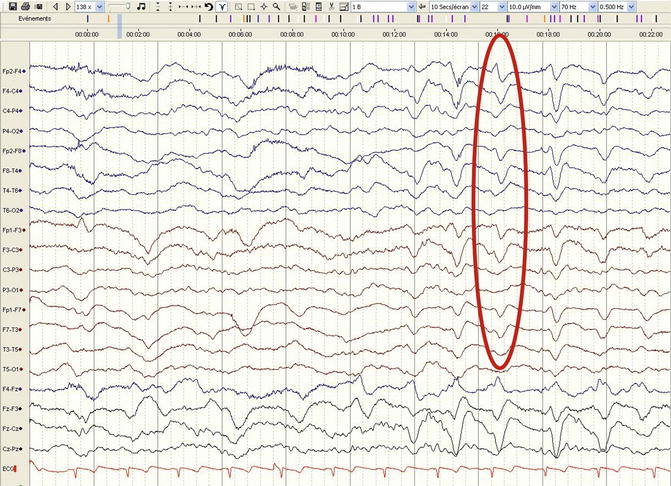

Fig. 5.1
Diffuse slowing and triphasic transients with a posteroanterior lag (red ellipse) in a 42-year-old man with a metabolic encephalopathy (longitudinal bipolar montage, 30 mm/s, 10 μV/mm)
5.2.3 Periodic Discharges
These represent one of the most common findings in the ICU setting and are labeled as generalized periodic [epileptiform] discharges (GPEDs or GPDs) and, if lateralized, PLEDs or LPDs (Table 5.4); since their presence does not necessarily represent an ongoing seizure, as they lay somewhere on the so-called ictal-interictal continuum, the term “epileptiform” should indeed better be avoided (Chong and Hirsch 2005; Hirsch et al. 2013). Importantly, transients with a triphasic appearance occurring (pseudo-) periodically also qualify for this definition. It has been recently reminded that these features are observed in patients with hypoxic-ischemic encephalopathy, ischemic stroke, hemorrhage, encephalitis, and metabolic disturbances; they tend to occur more often in patients showing also electrographic seizures (occurring in about 45–70 % of those with periodic discharges), but, somewhat counterintuitively, the impact on prognosis is not uniformly found: some authors recognize an independent association with poor outcome (Oddo et al. 2009), while others do not (Ong et al. 2012; Foreman et al. 2012), as opposed to the underlying EEG background reactivity (Ong et al. 2012).
5.2.4 Rhythmic Delta Activity
The summarizing eponym is RDA, but these features are also commonly labeled as frontal intermittent, rhythmic delta activity (FIRDA; see Table 5.4) because of the frequently observed anterior predominance. This EEG pattern is common and usually reactive to stimuli. Apart from the fact that symmetric rhythmic delta is not related to epilepsy, it represents a rather unspecific finding seen in patients with various structural brain lesions, toxic-metabolic disorders, brain infections, as well as other etiologies (Accolla et al. 2011; Sutter et al. 2013a). Symmetric delta slowing does not have any localizing value, but a marked asymmetric appearance may be associated with an underlying ipsilateral lesion (Accolla et al. 2011). As compared to triphasic waves and severe, diffuse EEG slowing, FIRDA seems to be related to a better outcome (Sutter et al. 2013a). Recently, the occurrence of lateralized rhythmic delta activity has been described in about 5 % of patients with impaired consciousness, with a prevalence of associated seizures similar to that observed with periodic discharges (Gaspard et al. 2013).
5.2.5 Stimulus-Induced Patterns
The first systematic description of stimulus-induced rhythmic, periodic, or ictal discharges (SIRPIDs; see Table 5.4 for the last proposed terminology) is just 10 years old (Hirsch et al. 2004). These patterns, arising following any stimulation on the lying, comatose patient, are not exceptional, with a reported prevalence of 22 % in the original description of a neuro-ICU cohort, encompassing all the etiological spectrum seen in this setting (Fig. 5.2); as the authors pointed out, a video correlation to recognize the stimuli (at times very subtle, such as a monitoring noise or caregiver’s steps beside the bed) is mandatory in order to differentiate these EEG features from spontaneous seizures. SIRPIDs may represent a heterogeneous EEG reaction that should not be regarded as a normal, physiological reactivity (the latter, as we have seen, is often related to a somewhat better prognosis): while some of these patterns clearly resemble electrographic seizures, others are not related to any ictal activity (Zeiler et al. 2011). Interestingly, SIRPIDs have received relatively little attention regarding their prognostic significance; recently, their occurrence in postanoxic patients undergoing hypothermia has however been related to poor outcome (Alvarez et al. 2013a).
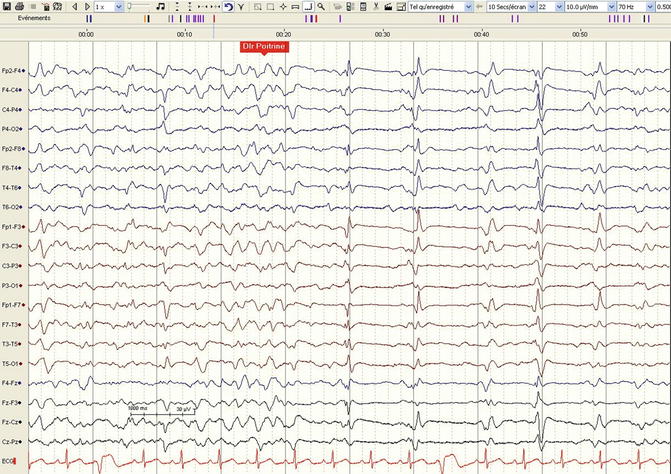

Fig. 5.2
Stimulus-induced rhythmic, periodic, or ictal discharges (SIRPIDs), in this case stimulus-induced generalized periodic discharges (SI-GPDs), in a 56-year-old man, during normothermia, 36 h after a cardiac arrest. The stimulation (pain applied to the chest) is marked in red (longitudinal bipolar montage, 30 mm/s, 10 μV/mm)
5.2.6 Electrographic Seizures
Seizures and status epilepticus represent a common challenge for caregivers in the ICU setting, and are mostly nonconvulsive (Claassen et al. 2004) (Chap. 1); the therapeutic criteria and approaches are discussed in Chaps. 3 and 4. Their specific role in terms of clinical prognosis varies among the etiologies and population studies; that is, it is difficult to identify a common pattern summarizing their impact independently from the etiology and the extent of active comorbidities.
Earlier observations that myoclonic status following resuscitation after cardiac arrest, often correlating with periodic EEG discharges, is linked to a poor outcome (Wijdicks et al. 1994) have been corroborated in patients treated with therapeutic hypothermia (Rossetti et al. 2007), particularly if electrographic seizures appear during cooling and despite pharmacological sedation (Rossetti et al. 2012). However, of note, prognosis does not prove invariably catastrophic for patients experiencing seizures after rewarming (Rossetti et al. 2009).
In patients with traumatic brain injury, seizures have also been independently associated with mortality (Hesdorffer et al. 2009), and it has been suggested that they aggravate cerebral damage (Vespa et al. 2007); similar findings also apply for subarachnoid hemorrhage (Claassen et al. 2006; Dennis et al. 2002), while in subjects with intracranial hemorrhage, although seizures have been associated with worse cerebral damage (Vespa et al. 2003), they do not seem to predict clinical prognosis independently (Passero et al. 2002; Claassen et al. 2007; Vespa et al. 2003). Acute seizures or status epilepticus in patients with ischemic stroke has been reported to be independently related to worse clinical outcome in hospital-based (very likely including a higher proportion of patients treated in the ICU) (Arboix et al. 2003; Knake et al. 2006) but not in population-based studies (Labovitz et al. 2001; Reith et al. 1997); globally, their occurrence is limited to about 2 % of the patients (Carrera et al. 2006). Patients with sepsis in the medical ICU are also subject to have (mostly nonconvulsive) seizures, which seem to correlate with worse prognosis (Oddo et al. 2009).
5.2.7 Alpha, Theta, and Spindle Coma
These EEG patterns are less frequent than the aforementioned; while they are mostly observed in comatose patients experiencing a cardiac arrest, they may also be found in subjects with toxic-metabolic disturbances, brain trauma, or infections (Kaplan et al. 1999, 2000). They are mainly defined by a dominant frequency and by a reversed spatial distribution, that is, the amplitude is usually higher in the frontal than in the posterior regions. The lack of reactivity to stimulations is regarded as characteristic by most (Young et al. 1997; Berkhoff et al. 2000) but not all authors (Kaplan et al. 1999).
Alpha and theta coma probably represent a common phenomenon: in fact, a transition among these frequencies has been described in several patients; alpha coma is usually seen at the low alpha band (7–8 Hz) (Synek and Synek 1984, 1988), and a progressive slowing leading to a diffuse EEG attenuation may be observed over some days (Fig. 5.3) in patients with poor prognosis. The mechanism is thought to be related to deafferentation of the cerebral cortex or cortical laminar necrosis or again drug intoxications (Kaplan et al. 1999). The presence of a reproducible variation of the background modulates the earlier assumption that alpha and theta coma invariably herald a poor outcome: the majority of patients showing a “reactive” alpha coma have been described to awaken, as opposed to those with no reactivity (Berkhoff et al. 2000; Kaplan et al. 1999). Spindle coma may reflect the preservation of thalamocortical loops following lesions located in the lower diencephalon, or in the brainstem, and therefore a lesser degree of brain dysfunction (Kaplan et al. 2000). This is illustrated by the fact that even if also in these cases background EEG reactivity plays a role for prognostic proposes, as opposed to alpha-theta coma, a considerable proportion of patients with nonreactive recordings may also awaken, thus conferring a better prognosis to this specific EEG pattern.
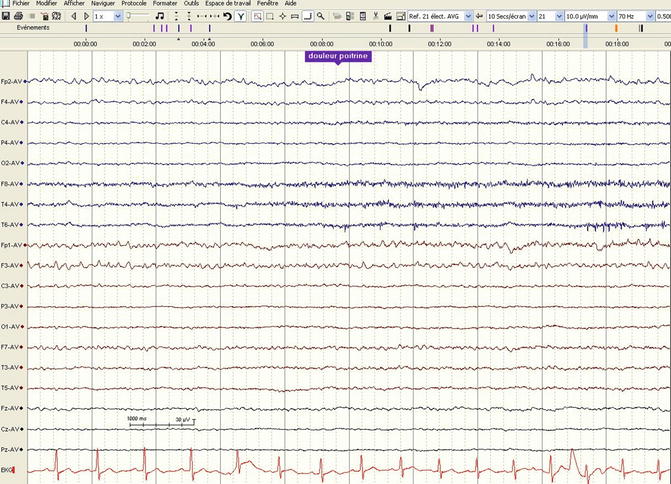

Fig. 5.3
Alpha-theta coma in a 43-year-old man, in normothermic conditions, 72 h after a cardiac arrest. The dominant frequency is 7 Hz, of higher amplitude in the frontal regions, without any reactivity to stimulations (pain applied to the chest, marked in blue; average referential montage, 30 mm/s, 10 μV/mm)
5.2.8 Sleep Spindles
While spindle coma is a pathologic condition with abundant spindles but lacking sleep-specific EEG features, the occurrence of physiological sleep patterns in patients with disorders of consciousness has recently been outlined as an important prognostic factor, likely reflecting the preservation of widespread brain regions and connections. This is nicely illustrated in patients with consciousness impairment following deep cerebral vein thrombosis involving the thalami and lacking spindles in the acute setting; the latter return upon resolution of the vasogenic thalamic edema (Rossetti et al. 2005). In subjects with severe traumatic brain injury (Urakami 2012; Landsness et al. 2011) and anoxic-ischemic encephalopathy (Landsness et al. 2011), the occurrence of K complexes and sleep spindles clearly correlates with a lesser degree of consciousness impairment (i.e., vegetative—or nonresponsive wakefulness—versus minimally conscious states). Of note, these studies have been conducted in a rehabilitation setting, several weeks after the initial insult, and an improvement in the spindle occurrence was described relatively late, up to 150 days (Urakami 2012); therefore, one should not automatically infer that the lack of physiological sleep in the acute setting portends the same dismal prognosis. In fact, during the first few days, acute brain changes and pharmacological sedation usually prevent the appearance of the specific sleep transients.
5.3 Particular Clinical Situations
In recent years, therapeutic hypothermia has experienced an increasing popularity, mainly in the context of anoxic-ischemic brain injury in neonates and adults (Holzer 2010). Core temperature lowering might lead to concerns regarding the EEG interpretation in these conditions; however, EEG changes in relation with cooling in patients undergoing hypothermic circulatory arrest for surgical purposes have been systematically analyzed. It is not until below 30 °C that periodic complexes appear; the temperature has to sink below 24 °C in order to observe diffuse intermittent suppression and below 18 °C for electrocerebral silence (Stecker et al. 2001). Hence, the common application of therapeutic hypothermia to 33 °C does not lead to marked EEG changes, apart from some mild slowing or amplitude attenuation that may also be induced by concomitant pharmacological sedation.
5.3.1 Hypoxic-Ischemic Encephalopathy: Adults
Outcome prognostication of comatose patients surviving a cardiac arrest has challenged clinicians for decades and has received increasing attention in these last years. The timing of assessment is critical, as electrophysiological evaluations within 12 (and maybe 24) h after the insult may lead to overestimation of the brain damage (Bassetti et al. 1996; Berkhoff et al. 2000), likely due to the acute, partially reversible neuronal electrical “shutdown.” This not only applies for patients in normothermia but also for those undergoing controlled temperature lowering (Alvarez et al. 2013b).
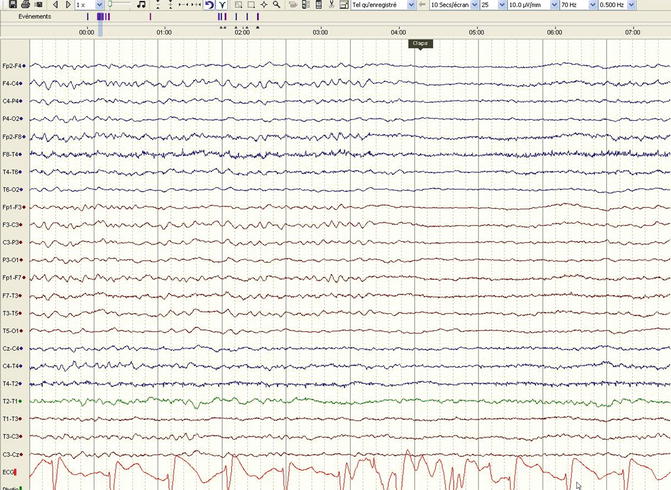
In this clinical setting, the prognostic role of the EEG is well established. In normothermia, patterns of monotonous, very diffuse low voltage or repetitive electric seizures or status epilepticus or periodic discharges without any identifiable background are considered to herald a poor prognosis (Fugate et al. 2010; Rossetti et al. 2010; Thenayan et al. 2010; Rittenberger et al. 2012; Wijdicks et al. 2006); the lack of background reactivity is also a reliable prognosticator (Rossetti et al. 2010; Thenayan et al. 2010). The EEG during therapeutic hypothermia has been recently described to provide very valuable prognostic information, not only regarding the continuity of the tracing (an isoelectric recording during hypothermia, 24 h after the cardiac arrest, is tightly related to non-awakening (Cloostermans et al. 2012)) but also lack of background reactivity: this feature, despite the concomitant use of moderate doses of sedation, has been related to a reliable forecast of poor outcome (Fig. 5.4) (Rossetti et al. 2012). However, a reactive EEG (even during hypothermia) does not necessarily imply a favorable prognosis: in fact, the occurrence of a discontinuous background and elevated biological markers (serum neuron-specific enolase) may point to patients with a less favorable outcome despite early reactivity (Tsetsou et al. 2013). In parallel, explorations of automated, amplitude-integrated EEG softwares have been described, pointing to the favorable prognostic role of a continuous signal, without electrographic status epilepticus (Rundgren et al. 2006, 2010); these approaches are, however, still not widely applied.