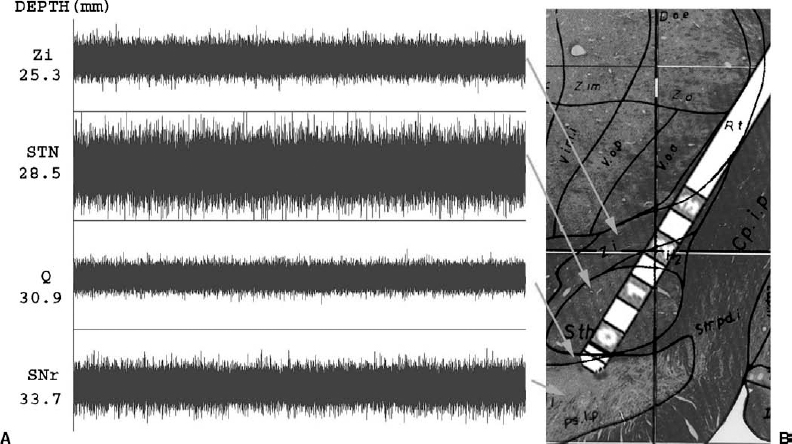
17 The efficacy of deep brain stimulation (DBS) for the treatment of movement disorders is well established. Thalamic stimulation for the treatment of tremor associated with Parkinson’s disease (PD) and essential tremor has been in widespread use for at least 15 years. More recently, the use of the subthalamic nucleus and the globus pallidus internalis (GPi) as targets for the treatment of the bradykinesia, dyskinesias, and on-off fluctuations associated with PD has become popular. The efficacy of DBS in these sites is incumbent upon three factors: (1) patient selection, (2) target localization for lead placement, and (3) programming of the stimulator. The first is beyond the scope of this book, and the second is covered in previous chapters. Although patient selection and lead placement may be the most important factors in determining outcome, successful treatment still requires careful selection of stimulation parameters. The goals for successful programming of a deep brain stimulator are threefold. First of all, the stimulation parameters must provide therapeutic benefit for the patient. Second, the stimulation should produce minimal if any side effects. Third, attempts should be made to select a program that provides maximal benefits for the patient with minimal power drain on the battery. Although this may seem the least important of the three, the cost associated with repeated surgery for generator replacement cannot be overlooked. In addition, each time the patient is subjected to an operation for replacement of the generator, there exists a risk of infecting the system. Despite its use for over two decades, little is known about the in vivo mechanism of action for DBS. Because high-frequency stimulation has demonstrated clinical effects comparable to lesioning, it has been postulated to exert its effect via a hyperpolarizing blockade of the cells within the target nucleus. However, work by Holsheimer et al1 and McIntyre and Grill2 would suggest that, at the pulse widths and amplitudes used in DBS, stimulation is likely affecting myelinated axons as opposed to cell bodies. Fibers running parallel to the direction of the stimulating current would be preferentially activated as opposed to those running transversely. In addition, given the short refractory period of myelinated axons, frequencies in the 100 to 200 Hz range are unlikely to lead to inhibition of conduction. However, what is not known is the response of the postsynaptic neuron to this frequency of stimulus. There is evidence to suggest that postsynaptic potentials may start to fuse at frequencies of 100 to 200 Hz.3 This synaptic depolarization can inactivate the spike-generating mechanism at the postsynaptic membrane, effectively inhibiting the postsynaptic cell. The relative contribution of this postsynaptic phenomenon remains unclear and does not take into account the potential effects of orthodromic stimulation on afferent fibers. Thus, although it appears that DBS preferentially stimulates axons, the ultimate effect it produces is difficult to predict. The variable volume of the neuropil that is affected by stimulation further confounds this. Based on animal data, average stimulation currents used in DBS may excite neural elements for a distance of 2 to 5 mm from the active cathode.4 As our clinical experience with deep brain stimulation continues to grow, our fundamental understanding of its effects at the cellular level unfortunately lags far behind. The process of deep brain stimulator programming can be time consuming and labor intensive in and of itself. In addition, patients frequently require concurrent adjustments in their medications. This can be particularly difficult soon after placement of the DBS electrodes, when microlesional effects are diminishing. For these reasons, at our institution, we have found that it is most efficient for the neurologists to conduct the majority of the programming. This allows for a single physician to manage both medical and stimulation therapies, which reduces the number of office visits for the patient and provides continuity of care. Patients are typically admitted to the hospital and undergo implantation of both the electrode and the generator during a single operation. They are then observed in the hospital for 1 to 2 days postoperatively. During this period, the system is typically programmed in a monopolar configuration at very low amplitudes. Some patients exhibit a microlesional effect from the implantation of the electrode and may not receive any initial programming. These patients are then scheduled for follow-up with neurology 1 to 2 weeks postoperatively for the initial programming session. This allows the patient to recover from surgery and provides enough time for the transient lesional effects to resolve. For their initial programming session, PD patients have their medication held for at least 12 hours. Baseline motor assessments are performed, evaluating the patient for rigidity, bradykinesia, gait, and postural stability. The electrode contacts are then sequentially evaluated in a monopolar configuration. Frequency and pulse width typically are kept at constant settings of 185 Hz and 90 msec, respectively. The amplitude is steadily increased to the tolerance level of the patient or until side effects occur. Repeat motor evaluation is then performed to assess efficacy of stimulation. This process is performed for each of the four electrode contacts. Ten to 15 minutes is allowed to pass between trials of separate contacts to allow for at least partial “washout” of any previous stimulation effects. If a satisfactory result cannot be achieved with monopolar stimulation, more complex arrays consisting of bipoles, tripoles, or multiple cathodes are tried. Once an effective program has been established, patients are given an appropriate dose of levodopa and are observed for dyskinesias. Further programming adjustments are made as required to treat any perceived drug-induced dyskinesias. The initial programming session, as described above, can take several hours. The process can be very taxing on patients, especially when they are kept in the medication-off state for prolonged periods. Knowledge of the exact position of electrode contacts relative to target structures, based on microelectrode recordings, may facilitate the programming process. Although no controlled studies exist demonstrating a benefit in outcome using MER for DBS electrode placement, let alone for DBS programming, we feel that the information obtained during microelectrode recordings is an additional tool to aid the clinician in the complex process of stimulator programming. At our institution, microelectrode recording (MER) is routinely performed on all patients undergoing electrode placement within the subthalamic nucleus or internal segment of the globus pallidus. These data are used to define the boundaries of the target nucleus and to identify adjacent structures. For example, during subthalamic nucleus surgery, recordings are made until the reticular portion of the substantia nigra is encountered; in the case of GPi surgery, recordings are performed until the subpallidal white matter is identified. Subsequent recording trajectories are performed as required to obtain a final pathway that traverses an adequate portion of the target nucleus. We do not routinely search for kinesthetic cells within the subthalamic nucleus or GPi, although this technique is advocated by some authors.5 Once the recordings are complete and an appropriate trajectory has been found, the lead is implanted. The lead is positioned so that the second (number 1) contact lies within the MER-defined center of the nucleus. For GPi, the most distal contact (number 0) is placed at the ventral border of the nucleus. We do not routinely conduct microelectrode recordings for placement of electrodes within the Vim of the thalamus. The consistency of targeting based on anatomical structures and the ease of performing intraoperative macrostimulation to assess efficacy have, in our experience, obviated the need for MER in these cases. However, many physicians still advocate the use of MER for the placement of Vim electrodes. Typically, the anterior and inferior borders of the ventrocaudal nucleus are identified by searching for cells responsive to tactile stimulation. Next, tremor cells are sought just anterior to this region, and a final lead is placed where macrostimulation produces maximum tremor suppression with minimal paresthesias. As stated above, the information obtained during microelectrode recordings allows the physician to define the borders of the target nucleus and to identify important neighboring structures. The first and most important application of these data are to ensure optimal lead placement, with a maximum number of contacts lying within the target structure. In addition, given the length of the electrode and the spacing of the contacts, this information can be used to identify the location of each contact relative to the nuclear borders and adjacent structures (Fig. 17–1). Armed with this knowledge, DBS programming can begin using those contacts, which are most likely to provide therapeutic benefit with minimal side effects. Should initial attempts at programming yield poor results, the physician is then able to refer back to the location of each contact with respect to the MER data and make appropriate adjustments.
Programming for DBS Using MER Data
LOUIS ANTHONY WHITWORTH
Physiological Effects of Stimulation
Deep Brain Stimulator Programming
Information Obtained during MER
Application of MER Data
Neupsy Key
Fastest Neupsy Insight Engine