Fig. 1
(a) Myelin basic protein (MBP) immunoreactivity in the ipsilateral caudate after a sham operation or a 100 μl blood injection (ICH) into the right caudate at 4 weeks after surgery, along with the time course of changes in MBP total area and number of bundles expressed as ipsilateral/contralateral ratios. Scale bar = 5 mm. (b) MBP immunoreactivity in the ipsi- and contralateral caudate in rats at 2 weeks after a sham operation or a 100 μl blood injection. Scale bar = 5 mm. Bar graphs displaying MBP total area and number of bundles in the ipsilateral caudate 2 weeks after 100 μl blood injection or a needle insertion. Values are means ± SD, #p < 0.01, *p < 0.05 by Student’s t-test
Treatment with ZnPP, delivered by intraperitoneal osmotic mini-pump, significantly attenuated the reduction in MBP positive area (0.82 ± 0.03 vs. 0.44 ± 0.04 in the vehicle, p < 0.01, Fig. 2a) and the number of bundles (0.87 ± 0.02 vs. 0.45 ± 0.02 in the vehicle, p < 0.01, Fig. 2b) at 28 days following ICH.
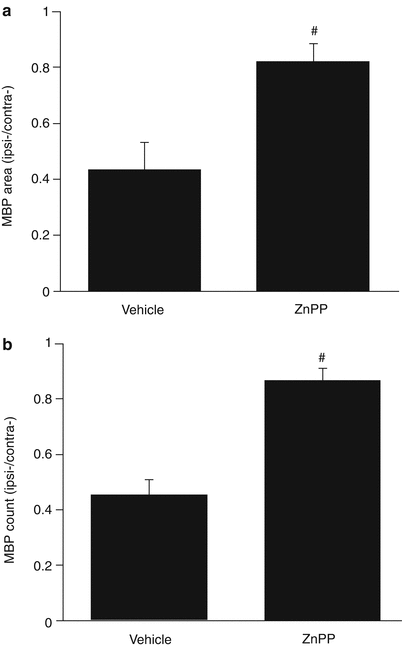

Fig. 2
Effects of ZnPP or vehicle treatment on (a) the myelin basic protein (MBP)-positive area and (b) the number of MBP-positive bundles in the ipsilateral caudate of rats 4 weeks after ICH. Values (± SD) are expressed as a ratio to the contralateral caudate. #p < 0.01 by Student’s t-test
Discussion
Intracerebral hemorrhage leads to secondary brain injury and severe neurological deficits. Such brain injury involves both gray and white matter [16]. However, most studies on experimental ICH-induced brain injury have focused on neuronal and gray matter alterations [6]. To date, few studies have quantified histological changes in white matter.
Oligodendrocytes are the major white matter cell type. MBP is produced by oligodendrocytes and it is the most abundant protein in the myelin sheath. Takahiro et al. [8] reported that myelin changes are the primary pathological event in cerebral white matter and the alteration in axons that occur with chronic hypoperfusion. In the present study, there was a time-dependent white matter injury after ICH. The decrease in both the number of MBP-labeled bundles in the ipsilateral caudate nucleus and their total area was almost 50 %. Degenerating myelin sheaths may be phagocytosed by macrophages.
The mechanisms of brain injury after ICH are complex. Hemoglobin and iron, its degradation product, are not only major factors responsible for acute brain edema formation and brain injury after ICH [4, 10, 16], but they also play a key role in persistent neurological deficits, even after the period when ICH-induced edema had resolved [3]. In the present study, we showed that ICH-induced white matter injury is progressive over the period of 1–28 days.
Heme oxygenases (HO) are key enzymes in heme degradation. There are three separate isozymes, HO-1, HO-2, and HO-3 [9]. HO-1 is markedly upregulated in the brain after ICH [4, 13, 15]. Our previous studies on HO inhibitors, including SnPP and ZnPP, showed that they can attenuate ICH- and hemoglobin-induced brain edema in rat ICH models [2, 4]. Similar results were found in a pig ICH model, with SnPP reducing both edema and clot volumes at 24 h after hematoma induction [14]. The present study shows that systemically administered ZnPP can markedly reduce myelin sheath injury after ICH and suggests that the brain protective effects of HO inhibitors are partly due to reductions in white matter injury.
In conclusion, progressive white matter injury occurs after ICH. ZnPP attenuated ICH-induced white matter injury, suggesting a role of heme degradation products in such damage.
Acknowledgment
This study was supported by grants NS-073595, NS-079157 and NS-084049 from the National Institutes of Health (NIH) and 973 Program-2014CB541600.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








