Psychopharmacological Treatment
 29.1 General Principles of Psychopharmacology
29.1 General Principles of Psychopharmacology
Psychopharmacologic advances continue to dramatically expand the parameters of psychiatric treatments. Greater understanding of how the brain functions has led to more effective, less toxic, better-tolerated, and more specifically targeted therapeutic agents. With the ever-increasing sophistication and array of treatment options, clinicians, however, must remain aware of potential adverse effects, drug–drug (and drug–food or drug–supplement) interactions, and how to manage the emergence of unwanted or unintended consequences. Newer drugs could ultimately lead to side effects that are not recognized initially. Keeping up with the latest research findings is increasingly important as these findings proliferate. A thorough understanding of the management of medication-induced side effects (either through treating the effect with another agent or substituting another primary agent) is necessary.
CLASSIFICATION
Medications used to treat psychiatric disorders are referred to as psychotropic drugs. These drugs are commonly described by their major clinical application, for example, antidepressants, antipsychotics, mood stabilizers, anxiolytics, hypnotics, cognitive enhancers, and stimulants. A problem with this approach is that, in many instances, drugs have multiple indications. For example, drugs such as the selective serotonin reuptake inhibitors (SSRIs) are both antidepressants and anxiolytics, and the serotonin-dopamine antagonists (SDAs) are both antipsychotics and mood stabilizers.
Psychotropic drugs have also been organized according to structure (e.g., tricyclic), mechanism (e.g., monoamine oxidase inhibitor [MAOI]), history (e.g., first generation, traditional), uniqueness (e.g., atypical), or indication (e.g., antidepressant). A further problem is that many drugs used to treat medical and neurological conditions are routinely used to treat psychiatric disorders.
In addition, psychotropic drug terminology can be confusing. The first pharmaceutical agents used to treat schizophrenia were termed tranquilizers. When newer drugs emerged as therapies for anxiety, a distinction was drawn between major and minor tranquilizers. At first, antidepressants were tricyclic antidepressants (TCAs) or MAOIs. In the 1970s and 1980s, as newer antidepressant drugs emerged, they were labeled as second- or third-generation antidepressants. More recently, older agents used as treatments for psychosis became known as typical, conventional, or traditional neuroleptics. Newer ones became atypical neuroleptics. In order to eliminate much of this confusion, in this section, drugs are presented according to shared mechanism of action or by similarity of structure to provide consistency, ease of reference, and comprehensiveness.
PHARMACOLOGICAL ACTIONS
Both genetic and environmental factors influence individual response to, and tolerability of, psychotropic agents. Thus, a drug that may not prove effective in many patients with a disorder can dramatically improve symptoms in others. In these cases, identification of characteristics that might predict potential candidates for that drug becomes important, but often remains elusive.
Drugs, even within the same class, are distinguished from one another by often subtle differences in molecular structure, types of interactions with neurotransmitter systems, differences in pharmacokinetics, the presence or absence of active metabolites, and protein binding. These differences, combined with the biochemistry of the patient, account for the profile of efficacy, tolerability, and safety and the risk-to-benefit ratio for the individual. These multiple variables, some poorly understood, make it difficult to predict a drug’s effect with certainty. Nevertheless, knowledge of the nature of each property increases the likelihood of successful treatment. The clinical effects of drugs are best understood in terms of pharmacokinetics, which describes what the body does to a drug, and pharmacodynamics, which describes what the drug does to the body.
Pharmacokinetics and pharmacodynamics need to be seen in the context of the underlying variability among patients with respect to how drug effects are expressed clinically. Patients differ in their therapeutic response to a drug and the experience of side effects. It is increasingly clear that these differences have a strong genetic basis. Pharmacogenetics research is attempting to identify the role of genetics in drug response.
DRUG SELECTION
Although all U.S. Food and Drug Administration (FDA)-approved psychotropics are similar in overall effectiveness for their indicated disorder, they differ considerably in their pharmacology and in their efficacy and adverse effects on individual patients. The ability of a drug to prove effective, thus, is only partially predictable and is dependent on poorly understood patient variables. Nevertheless, it is possible that some drugs have a niche in which they can be uniquely helpful for a subgroup of patients, without demonstrating any overall superiority in efficacy. No drug is universally effective, and no evidence indicates the unambiguous superiority of any single agent as a treatment for any major psychiatric disorders. The only exception, clozapine (Clozaril), has been approved by the FDA as a treatment for cases of treatment-refractory schizophrenia.
Decisions about drug selection and use are made on a case-by-case basis, relying on the individual judgment by the physician. Other factors in drug selection are the characteristics of the drug and the nature of the patients illness. Each of these components affects the probability of a successful outcome.
DRUG FACTORS
Pharmacodynamics
The time course and intensity of a drug’s effects are referred to as its pharmacodynamics. Major pharmacodynamic considerations include receptor mechanisms, the dose–response curve, the therapeutic index, and the development of tolerance, dependence, and withdrawal phenomena. Drug mechanism of action is subsumed under pharmacodynamics. The clinical response to a drug, including adverse reactions, results from an interaction between that drug and a patient’s susceptibility to those actions. Pharmacogenetic studies are beginning to identify genetic polymorphisms linked to individual differences in treatment response and sensitivity to side effects.
Mechanisms
The mechanisms through which most psychotropic drugs produce their therapeutic effects remain poorly understood. Standard explanations focus on ways that drugs alter synaptic concentrations of dopamine, serotonin, norepinephrine, histamine, γ-aminobutyric acid (GABA), or acetylcholine. These changes are said to result from receptor antagonists or agonists, interference with neurotransmitter reuptake, enhancement of neurotransmitter release, or inhibition of enzymes. Specific drugs are associated with permutations or combinations of these actions. For example, a drug can be an agonist for a receptor, thus stimulating the specific biological activity of the receptor, or an antagonist, thus inhibiting the biological activity. Some drugs are partial agonists, because they are not capable of fully activating a specific receptor. Some psychotropic drugs also produce clinical effects through mechanisms other than receptor interactions. For example, lithium (Eskalith) can act by directly inhibiting the enzyme inositol-1-phosphatase. Some effects are closely linked to a specific synaptic effect. For example, most medications that treat psychosis share the ability to block the dopamine type 2 (D2) receptor. Similarly, benzodiazepine agonists bind a receptor complex that contains benzodiazepine and GABA receptors.
Further illustrating the fact that the mechanisms of action of psychotropic drugs remain only partially understood are observations that medications that do not directly target monoamine neurotransmitters can be remarkably effective in treating some psychiatric disorders. For example, ketamine (Ketalar), an anesthetic agent that targets glutamate, can rapidly and dramatically alleviate symptoms of depression when given as a slow infusion. Another example involves the antibiotic minocycline (Solodyn), which has been shown to have antidepressant effects. Along with other findings, this suggests that the immune system and inflammatory responses may underlie some mood disorders.
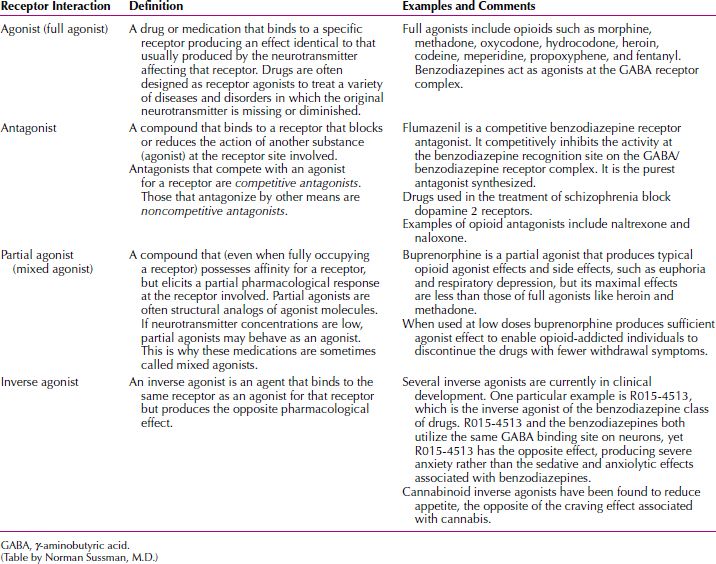
Accounts of so-called mechanisms of action should nevertheless be kept in perspective. Explanations of how psychotropic drugs actually work that focus on synaptic elements represent an oversimplification of a complex series of events. If merely raising or lowering levels of neurotransmitter activity is associated with the clinical effects of a drug, then all drugs that cause these changes should produce equivalent benefits. This is not the case. Multiple obscure actions, several steps removed from events at neuronal receptor sites, are probably responsible for the therapeutic effects of psychotropic drugs. These downstream elements are postulated to represent the actual reasons that these drugs produce clinical improvement. A glossary of terms related to receptor drug interactions is given in Table 29.1-1.
 Table 29.1-1
Table 29.1-1
Glossary of Receptor Drug Interactions
SIDE EFFECTS
Side effects are an unavoidable risk of medication treatment. Although it is impossible to have an encyclopedic knowledge of all possible adverse drug effects, prescribing clinicians should be familiar with the more common adverse effects, as well as those with serious medical consequences. No single text or document, including the product information, contains a complete list of possible treatment-emergent events.
Side effect considerations include the probability of its occurrence, its impact on a patient’s quality of life, its time course, and its cause. Just as no one drug is certain to produce clinical improvement in all patients, no side effect, no matter how common, occurs in every patient. When concurrent medical disorders or a history of a similar adverse reaction puts a patient at increased risk for a side effect, it is logical to consider prescribing a compound not typically associated with that adverse reaction.
Side effects can result from the same pharmacological action that is responsible for a drug’s therapeutic activity or from an unrelated property. In examples of the latter, some of the most common adverse effects of the TCAs are caused by blockade of muscarinic acetylcholine receptors or histamine 2 receptors. If a patient is sensitive to these effects, alternative agents without these properties should be prescribed. When side effects are manifestations of the drug’s presumed mechanism of action, side effects may be unavoidable. Thus, blockade of serotonin reuptake by SSRIs can cause nausea and sexual dysfunction. The D2 blockade of drugs used to treat psychosis can cause extrapyramidal side effects. Agonist action of benzodiazepine receptors can cause ataxia and daytime sleepiness. In these cases, additional medications are frequently used to make the primary agent better tolerated.
Time Course
Adverse effects differ in terms of their onset and duration. Some side effects appear at the outset of treatment and then rapidly diminish. Nausea occurring with SSRIs or venlafaxine (Effexor) and sedation occurring with mirtazapine (Remeron) are good examples of early, time-limited side effects. Early-onset, but persistent, side effects include dry mouth that is associated with noradrenergic reuptake inhibition or antimuscarinic activity. Some side effects appear later in treatment (late-appearing side effects) and, sometimes, may be just the opposite of adverse events early in treatment. For example, patients may typically lose weight during early treatment with SSRIs, only to find, over time, a reversal occurs, so that they gain weight. Similarly, early activation or agitation may be followed by constant fatigue or apathy. Because most data about new drugs come from short-term studies, generally 8 weeks in duration, early-onset side effects are overrepresented in product information and descriptions of newly marketed information. It is essential that clinicians follow the letters to the editor sections of journals and other sources of information to update their understanding of the true side effect profile of a drug.
Adverse effects differ in their impact on compliance and potential to cause harm. Depending on a patient’s threshold of tolerance for a side effect and the impact on quality of life, side effects can lead to drug discontinuation. Examples of serious side effects include agranulocytosis (clozapine), Stevens-Johnson syndrome (lamotrigine [Lamictal]), hepatic failure (nefazodone [Serzone]), stroke (phenelzine [Nardil]), and heart block (thioridazine [Mellaril]). Overall, the risk of life-threatening side effects with psychotropics is low. Drugs that carry such a risk should be monitored more closely, and the prescribing physician should take into account whether the potential clinical benefits justify the additional risk. Any drug with a serious risk, as reflected in a black box warning, is generally used less extensively than would otherwise be the case.
In the case of haloperidol (Haldol) and other dopamine receptor antagonists, long-term complications, such as tardive dyskinesia, have been well documented. Emerging evidence also suggests that the use of dopamine antagonists is associated with a small increase in the risk of breast cancer and that this is related to larger cumulative doses. In cases in which serious risk is associated with a drug, closer medical monitoring of medication treatment is warranted. Because the most widely used psychotropics, such as the SSRIs and serotonin-dopamine antagonists, have only been in use since the 1980s or 1990s, there is less certainty about long-term effects, but no evidence indicates that side effects are not merely extensions of those already evident during initial therapy. It should also be kept in mind that most drugs used in the treatment of chronic medical disorders have not been in use sufficiently long to provide assurances about unintended long-term adverse effects.
Suicidal Ideation and Antidepressant Treatment
The issue of antidepressant-associated suicide has become front-page news, the result of an analysis suggesting a link between medication use and suicidal ideation among children, adolescents, and adults up to age 24 in short-term (4 to 16 weeks), placebo-controlled trials of nine newer antidepressant drugs. The data from trials involving more than 4,400 patients suggested that the average risk of suicidal thinking or behavior (suicidality) during the first few months of treatment in those receiving antidepressants was 4 percent, twice the placebo risk of 2 percent. No suicides occurred in these trials. The analysis also showed no increase in suicide risk among the 25 to 65 age group. Antidepressants reduced suicidality among those over age 65.
Following public hearings on the subject, in October 2004, the FDA requested the addition of black box warnings—the most serious warning placed on the labeling of a prescription medication—to all antidepressant drugs, old and new. This action raised alarm among parents and physicians and prompted an explosion of advertisements by malpractice attorneys. Most important, antidepressant prescriptions written for adolescents declined, whereas those for adults flattened, after years of growth.
A large study of real world patients published in the January 2006 issue of the American Journal of Psychiatry raised serious doubt about true antidepressants and suicidality and about the wisdom of the FDA’s decision to change the labeling. The study examined suicides and hospitalizations for suicide attempts in the medical records of 65,103 members of a nonprofit insurer in the Pacific Northwest that covers about 500,000 people who received antidepressants from 1992 to 2003. It found that (1) newer antidepressants were associated with a more rapid and greater reduction in risk than older types of antidepressants and (2) patients were significantly more likely to attempt or commit suicide in the month before they began drug therapy than in the 6 months after starting it.
This is not the first time credible evidence has contradicted a significant link between antidepressant use and increased risk of suicide. At the hearings that led to the black box warning, John Mann of Columbia University presented population data showing that since 1987, the year before fluoxetine (Prozac) became the first marketed SSRI, suicide rates in the United States began dropping, and that areas in the United States with the highest SSRI prescription rates had the biggest decline in suicides. For every 10 percent increase in prescription rates, the US suicide rate declined 3 percent.
Another study, a review of 588 case files of patients aged 10 to 19, found that a 1 percent increase in antidepressant use was associated with a decrease of 0.23 suicides per 100,000 adolescents per year.
A more important question, given how slight the risk may be, if indeed it exists, is whether as a result of the FDA’s ill-considered actions, some depressed patients are not getting potentially life-saving treatment. Epidemiological findings from several countries, including the United States, have shown that decreased prescribing of antidepressants for depressed children and adolescents resulted in an increase in suicide rates in those populations.
Side Effects Associated with Newer Medications
All medications are associated with side effects. The clinician should be aware of these, be able to recognize them, and take appropriate measures to treat them.
Somnolence. Sedation is often an intended effect of many psychotropic drugs, especially when used to treat insomnia, anxiety, or agitation. Daytime sleepiness, or somnolence, is also an unwanted adverse event, however. It is important for the clinician to alert patients to the possibility of sedation and to document that the person was advised to exercise caution when operating any type of vehicle or mechanical equipment. Some somnolence results from a carryover of nighttime use of drugs as hypnotics. Even with drugs, such as the SSRIs, which are activating to many patients, somnolence can be problematic. In some instances, it results from impairment of sleep quality. Chronic use of SSRIs can cause some patients to experience a subjective sense of fatigue, exhaustion, or yawning, even with adequate amounts of sleep. Management of unwanted somnolence includes adjustment of dose or timing of administration, switching to alternative medications, addition of small doses of stimulants, or the addition of modafinil (Provigil).
Gastrointestinal Disturbances. The major gastrointestinal (GI) side effects of the older antidepressant and antipsychotic drugs consisted primarily of constipation and dry mouth, a consequence of their antimuscarinic activity. Most of the newer drugs have little antimuscarinic activity, but do have effects on the serotonin system. Most of the body’s serotonin is in the GI tract, and serotonergic drugs often cause varying degrees of stomach pain, nausea, flatulence, and diarrhea. In most cases, these side effects are transient, but some persons never accommodate and must switch to another class of drugs. Initial use of lower doses or use of delayed release preparations are the most effective strategies for minimizing GI side effects.
Movement Disorders. The introduction of serotonin-dopamine antagonists has greatly reduced the incidence of medication-induced movement disorders, but varying degrees of dose-related parkinsonism, akathisia, and dystonia still occur. Risperidone (Risperdal) most closely resembles the older agents in terms of these side effects. Olanzapine (Zyprexa) also causes more extrapyramidal effects than clinical trials suggested. Aripiprazole (Abilify) causes severe akathisia. There have been rare reports of SSRI-induced movement disorders, ranging from akathisia to tardive dyskinesia.
Sexual Dysfunction. The use of psychiatric drugs can be associated with sexual dysfunction—decreased libido, impaired ejaculation and erection, and inhibition of female orgasm. In clinical trials with the SSRIs, the extent of sexual side effects was grossly underestimated, because data were based on spontaneous reports by patients. The rate of sexual dysfunction in the original fluoxetine product information, for example, was less than 5 percent. In subsequent studies in which information about sexual side effects was elicited by specific questions, the rate of SSRI-associated sexual dysfunction was found to be between 35 and 75 percent. In clinical practice, patients are not likely to report sexual dysfunction spontaneously to the physician, so it is important to ask about this side effect. Also, some sexual dysfunctions may be related to the primary psychiatric disorder. Nevertheless, if sexual dysfunction emerges after pharmacotherapy has begun and the primary response to treatment has been positive, it may be worthwhile to attempt to treat the symptoms. Long lists of possible antidotes to these side effects have evolved, but few interventions are consistently effective, and few have more than anecdotal evidence to support their use. The clinician and patient should consider the possibility of sexual side effects with a patient when selecting a drug and switching treatment to another drug that is less or not at all associated with sexual dysfunction if this adverse effect is not acceptable to the patient.
Weight Gain. Weight gain accompanies the use of many psychotropic drugs as a result of retained fluid, increased caloric intake, decreased exercise, or altered metabolism. Weight gain can also occur as a symptom of disorder, as in bulimia or atypical depression, or as a sign of recovery from an episode of illness. Treatment-emergent increase in body weight is a common reason for noncompliance with a drug regimen. No specific mechanisms have been identified as causing weight gain, and it appears that the histamine and serotonin systems mediate changes in weight associated with many drugs used to treat depression and psychosis. Metformin (Glucophage) has been reported to facilitate weight loss among patients whose weight gain is attributed to use of serotonin-dopamine reuptake inhibitors and valproic acid (Depakene). Valproate (Depacon), as well as olanzapine, has been linked to the development of insulin resistance, which could induce appetite increase, with subsequent weight increase. Weight gain is a noteworthy side effect of clozapine (Clozaril) and olanzapine. Genetic factors that regulate body weight, as well as the related problem of diabetes mellitus, seem to involve the 5-HT2C receptor. There is a genetic polymorphism of the promoter region of this receptor, with significantly less weight gain in patients with the variant allele than in those without this allele. Drugs with a strong 5-HT2C affinity would be expected to have a greater impact on body weight of patients with a polymorphism of the 5-HT2C receptor promoter region.
Weight Loss. Initial weight loss is associated with SSRI treatment but is usually transient, with most weight being regained within the first few months. Bupropion (Wellbutrin) has been shown to cause modest weight loss that is sustained. When combined with diet and lifestyle changes, bupropion can facilitate more significant weight loss. Topiramate (Topamax) and zonisamide (Zonegran), marketed as treatments for epilepsy, sometimes produce substantial, sustained loss of weight.
Glucose Changes. Increased risk of glucose abnormalities, including diabetes mellitus, is associated with weight increase during psychotropic drug therapy. Clozapine and olanzapine are associated with a greater risk than other serotonin-dopamine antagonists of abnormalities in fasting glucose levels, as well as hyperosmolar diabetes and ketoacidosis. This dysregulation of glucose homeostasis appears to be drug induced and increases glucagon.
Hyponatremia. Hyponatremia is associated with oxcarbazepine (Trileptal) and SSRI treatment, especially in elderly patients. Confusion, agitation, and lethargy are common symptoms.
Cognitive Impairment. Cognitive impairment means a disturbance in the capacity to think. Some agents, such as the benzodiazepine agonists, are recognized as causes of cognitive impairment. Other widely used psychotropics, such as the SSRIs, lamotrigine (Lamictal), gabapentin (Neurontin), lithium, TCAs, and bupropion, however, are also associated with varying degrees of memory impairment and word-finding difficulties. In contrast to the benzodiazepine-induced anterograde amnesia, these agents cause a more subtle type of absent-mindedness. Drugs with anticholinergic properties are likely to worsen memory performance.
Sweating. Severe perspiration unrelated to ambient temperature is associated with TCAs, SSRIs, and venlafaxine. This side effect is often socially disabling. Attempts can be made to treat this side effect with alpha agents, such as terazosin (Hytrin) and oxybutynin (Ditropan).
Cardiovascular Disturbances. Newer agents are less likely to have direct cardiac effects. Many older agents, such as TCAs and phenothiazines, affected blood pressure and cardiac conduction. Thioridazine (Mellaril), which has been in use for decades, has been shown to prolong the QTc interval in a dose-related manner and may increase the risk of sudden death by delaying ventricular repolarization and causing torsades de pointes. Newer drugs are now routinely scrutinized for evidence of cardiac effects. A promising treatment for psychosis, sertindole (Serlect), was not marketed because the FDA would have required a black box warning. Slight QTc effects noted with ziprasidone (Geodon) delayed the marketing of that drug. Clozapine can cause myocarditis in rare cases of which the clinician should be aware.
Rash. Any medication is a potential source of a drug rash. Some psychotropics, such as carbamazepine (Equetro, Tegretol) and lamotrigine, have been linked to an increased risk of serious exfoliative dermatitis. Commonly referred to as Stevens-Johnson syndrome, this condition is a systemic, immune-mediated reaction that can prove fatal or result in permanent scarring or blindness. All patients should be informed about the potential seriousness of lesions that are widespread, that occur above the neck, that involve the mucous membranes, and that may be associated with fever and lymphadenopathy. If such symptoms manifest, a patient should be instructed at the time that the medication is prescribed to go immediately to an emergency department.
Idiosyncratic and Paradoxical Drug Responses
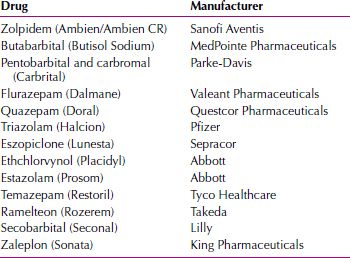
Idiosyncratic reactions occur in a very small percentage of patients taking a drug. The reactions are not related to the known pharmacologic properties, and most likely represent a genetically based abnormal sensitivity to a drug. A paradoxical response represents the manifestation of a clinical effect the opposite of what is expected. In March 2007, the FDA reported dissociative-like states associated with certain sedative hypnotics. These included behaviors such as sleepwalking, binge eating, aggressive outbursts, and night driving of which the patient was unaware. Table 29.1-2 lists the drugs required to have warning labels for that effect.
 Table 29.1-2
Table 29.1-2
Sedative Hypnotics Cited by the U.S. Food and Drug Administration
Therapeutic Index
Therapeutic index is a relative measure of the toxicity or safety of a drug and is defined as the ratio of the median toxic dose to the median effective dose. The median toxic dose is the dose at which 50 percent of patients experience a specific toxic effect, and the median effective dose is the dose at which 50 percent of patients have a specified therapeutic effect. When the therapeutic index is high, as it is for haloperidol, it is reflected by the wide range of dosages in which that drug is prescribed. Conversely, the therapeutic index for lithium is quite low, thus requiring careful monitoring of serum lithium levels in patients for whom the drug is prescribed.
Overdose
Safety in overdose is always a consideration in drug selection. Almost all of the newer agents, however, have a wide margin of safety when taken in overdose. By contrast, a 1-month supply of TCAs could be fatal. The depressed patients they were used to treat are the group most at risk to attempt suicide. Because even the safest drugs can sometimes produce severe medical complications, especially when combined with other agents, clinicians must recognize that the prescribed medication can be used in an attempt to commit suicide. Although it is prudent to write nonrefillable prescriptions for small quantities, this practice passes along increased copay costs to the patient. In fact, many pharmacy benefit management programs encourage the prescribing of a 3-month supply of medication.
In cases in which suicide is a major concern, an attempt should be made to verify that the medication is not being hoarded for a later overdose attempt. Random pill counts or asking a family member to dispense daily doses may be helpful. Some patients attempt suicide just as they are beginning to recover. Large quantities of medications with a low therapeutic index should be prescribed judiciously. Another reason to limit the number of pills prescribed is the possibility of accidental ingestion of medications by children in the household. Psychotherapeutic medications should be kept in a safe place.
Physicians who work in emergency rooms should know which drugs can be hemodialyzed. The issues involved are complex and are not based on any single chemical property of the drug. For example, it is generally presumed that drugs with low protein binding are good candidates for dialysis. Venlafaxine, however, is only 27 percent protein bound and is too large as a molecule dialyzed. Hemodialysis is effective for treating overdose of valproic acid.
Pharmacokinetics
Pharmacokinetic drug interactions are the effects of drugs on the plasma concentrations of each other, and pharmacodynamic drug interactions are the effects of drugs on the biological activities of each other. Pharmacokinetic concepts are used to describe and predict the time course of drug concentrations in different parts of the body, such as plasma, adipose tissue, and the central nervous system (CNS). From a clinical perspective, pharmacokinetic methods help explain or predict the onset and duration of drug activity and interactions between drugs that alter their metabolism or excretion.
Pharmacogenetic research focuses on finding variant alleles that alter drug pharmacokinetics and pharmacodynamics. Researchers are attempting to identify genetic differences in how enzymes metabolize psychotropics, as well as CNS proteins directly involved in drug action. Likely, identification of patient genotypes will facilitate prediction of clinical response to different types of drugs.
Most clinicians need to consult charts or computer programs to determine when potential interactions may occur and, if so, how clinically relevant they may be. Whenever possible, it is preferable to use a medication that produces minimal risk of drug interactions. Also, it is recommended that prescribers know the interaction profiles of the drugs they most commonly prescribe.
Examples of pharmacokinetic interactions include one drug increasing or decreasing the concentrations of a coadministered compound. These types of interactions can also lead to altered concentrations of metabolites. In some cases, there may also be interference with the conversion of a drug to its active metabolite. Enormous variability exists among patients with respect to pharmacokinetic parameters, such as drug absorption and metabolism. Another type of interaction is represented by interactions involving the kidney. Commonly used medications, such as angiotensin-converting enzyme (ACE) inhibitors, nonsteroidal anti-inflammatory drugs (NSAIDs), and thiazides, decrease renal clearance of lithium, increasing the likelihood of severe elevations of lithium. Drug interactions can occur pharmacokinetically or pharmacodynamically.
Pharmacogenetics is being used to study why patients differ in the way they metabolize drugs. In patients who are ultrarapid or extensive metabolizers, the concentrations of a drug may be lower than expected.
PATIENT-RELATED FACTORS
Response to medication and sensitivity to side effects are influenced by factors related to the patient. This is why there is no one-size-fits-all approach to pharmacological treatment. Patient-related variables include diagnosis, genetic factors, lifestyle, overall medical status, concurrent disorders, and history of drug response. A patient’s attitude toward medication in general, aversion to certain types of side effects, and preference for a specific agent also need to be considered.
Diagnosis
Failure to correctly diagnose a disorder diminishes the likelihood of optimal drug selection. Misdiagnosis not only can result in a missed opportunity, but it also can, at times, produce worsening of symptoms. Inadvertently diagnosing a patient in the depressed phase of bipolar disorder as having unipolar depression can induce mania or rapid cycling. Treatment failure or exacerbation of symptoms should prompt a reassessment of the working diagnosis.
Past Treatment Response
A specific drug should be selected according to the patient’s history of drug response (compliance, therapeutic response, adverse effects), the patient’s family history of drug response, the profile of adverse effects for that drug with regard to the particular patient, and the prescribing clinician’s usual practice. If a drug has previously been effective in treating a patient or a family member, the same drug should be used again. For reasons that are not understood, however, some patients fail to respond to a previously effective agent when challenged again. A history of severe adverse effects from a specific drug is a strong indicator that the patient would not be compliant with that particular drug.
It is helpful if patients can recall the details of past psychotropic drug treatment: the drugs prescribed, in what dosages, for how long, and in what combinations. Because of their mental disorders, many patients, however, are poor historians. If possible, patients’ medical records should be obtained to confirm their reports. Family members are a good source of collateral information.
Response in Family Members
It is widely held that drug responses cluster in families. Thus, response to a drug in a relative is an indicator of whether a patient might also benefit from that medication. Although no conclusive evidence supports this as a consideration in drug selection, existing studies do confirm that a history of positive response to treatment with a drug should be considered in making treatment decisions.
Concurrent Medical or Psychiatric Disorders
Initial assessment should elicit information about coexisting medical disorders. In some cases, a medical disorder may be responsible for the symptoms. Patients with thyroid disease who are not adequately treated may appear depressed. Sleep apnea produces depression and cognitive impairment. Rare conditions, such as Kleine-Levin syndrome, can mimic bipolar disorder. A drug should be selected that minimally exacerbates any preexisting medical problems that a particular patient may have.
Recreational drug use, excessive consumption of alcohol, and frequent ingestion of caffeine-containing beverages can complicate and even undermine psychotropic drug treatment. These compounds possess significant psychoactive properties and, in some cases, may represent the source of the patient’s symptoms. It is reasonable to ask patients to abstain from use of these substances, at least until the benefits of psychotropic drug treatment have been unequivocally established. Gradual reintroduction of moderate amounts of alcohol, tea, and coffee can then take place. Patients can then observe for themselves whether there are any untoward effects on their clinical status.
INFORMED CONSENT AND PATIENT EDUCATION
Establishing trust and providing motivation to comply with the medication regimen are essential components of successful treatment. Patients should be informed about treatment options and the probable side effects and unique benefits of each treatment. Patient preference should be respected, unless a compelling advantage exists involving efficacy, tolerability, or safety with an alternative agent. If a particular medication is being recommended, the reasons for this recommendation should be explained. Patients are more likely to continue taking their medication if they fully understand the reasons why it is being prescribed.
A strong therapeutic alliance between a clinician and a patient is always helpful. Given the unpredictability of medication response, the frequent occurrence of side effects, and underlying ambivalence about, or fear of taking, medication, a positive, trusting relationship serves to improve patient compliance. Repeated failed trials may be needed before a response is seen. A patient’s confidence in the physician’s knowledge and judgment enables medication trials and more complex regimens, such as the use of multiple medications.
Discussions about drug selection should be documented in notes, but a signed informed consent is not needed. Surprisingly, patients who are informed of potential adverse effects report a higher incidence of side effects but do not have higher rates of premature discontinuation.
How the patient and family are engaged in the treatment plan can determine the success of treatment. The psychodynamic meaning of pharmacotherapy to the patient and family and environmental influences, psychosocial stressors, and support should be explored. Some patients may view drug treatment as a panacea, and others may view it as the enemy. With the patient’s consent, relatives and other clinicians should be instructed about the reasons for the drug treatment, as well as the expected benefits and potential risks.
DOSING, DURATION, AND MONITORING
Dosing
The clinically effective dose for treatment depends on the characteristics of the drug and patient factors, such as inherited sensitivity and ability to metabolize a drug, concurrent medical disorders, use of concurrent medications, and history of exposure to previous medications.
Plasma concentrations of many psychotropics can vary up to tenfold. Thus, to some extent, the optimal dose for an individual is ultimately determined by trial and error, guided by the empirical evidence of the usual dose range for that drug. In some cases, it may prove helpful to test patients for genetic polymorphisms involving hepatic enzymes. Patients who are ultrarapid metabolizers of certain drugs may require higher than normal dosing. Slow metabolizers might demonstrate side effects and even toxicity at very low doses.
Some drugs demonstrate a clear relationship between increases in dose and clinical response. This dose–response curve plots the drug concentration against the effects of the drug.
The potency of a drug refers to the relative dose required to achieve certain effects, not to its efficacy. Haloperidol, for example, is more potent than chlorpromazine (Thorazine), because approximately 5 mg of haloperidol is required to achieve the same therapeutic effect as 100 mg of chlorpromazine. These drugs, however, are equal in their clinical efficacy—that is, the maximal clinical response achievable by administration of a drug.
Drugs must be used in effective dosages for sufficient periods. Although drug tolerability and safety are always considerations, subtherapeutic doses and incomplete therapeutic trials should be avoided. The use of inadequate doses merely exposes the patient to the risk of side effects, without providing the probability of therapeutic benefit. In view of the wide margin of safety associated with most currently prescribed medications, more risk exists in underdosing than in overshooting the recommended dose range.
Time of dosing is usually based on the plasma half-life of a drug and its side effect profile. Sedating drugs are given all at night or with disproportionate daily doses at night. The opposite is true with activating drugs. The frequency of dosing is less clear-cut. Most dosing regimens of psychotropic drugs, such as once-a-day versus divided doses, are based on measurements of plasma concentrations rather than receptor occupancy in the brain. Evidence suggests a significant dissociation exists between brain and plasma kinetics. Reliance on plasma kinetics as the basis for dosing regimens leads to misunderstanding of necessary schedules.
As a rule, psychotropic drugs should be used continuously. Exceptions are the use of drugs for insomnia, acute agitation, and severe situational anxiety. A common mistake is the use of high-potency benzodiazepines, such as alprazolam (Xanax) and clonazepam (Klonopin), only after an attack has begun. These drugs should be used as part of a regular schedule to prevent attacks.
Some patients who experience sexual dysfunction while being treated with SSRIs take a drug holiday, that is, they skip a daily dose from time to time to facilitate sexual performance.
Intermittent dosing regimens of SSRIs have been found to be effective as a treatment for premenstrual dysphoric disorder. The drugs are taken daily during the 2-week luteal phase of the menstrual cycle.
Duration of Treatment
A common question from a patient is “How long do I need to take the medication?” The answer depends on multiple variables, including the nature of the disorder, the duration of symptoms, the family history, and the extent to which the patient tolerates and benefits from the medication. Patients can be given a reasonable explanation of the probabilities but should be told that it is first best to see if the medication works for him or her and whether any side effects are acceptable. Any more definitive discussion of treatment duration can be held once the degree of success is clear. Even patients with a philosophical aversion to the use of psychotropic drugs may elect to stay on medication indefinitely if the magnitude of improvement is great. Most psychiatric disorders have high rates of chronicity and relapse. Because of this, long-term treatment is often needed to prevent recurrence. Nevertheless, the fact remains that psychotropic drugs are not said to cure the disorders they treat but rather to help control the symptoms.
Treatment is conceptually broken down into three phases: the initial therapeutic trial, the continuation, and the maintenance phase. The initial period of treatment should last at least several weeks because of the delay in therapeutic effects that characterizes most classes of psychotropic drugs. The required duration of a therapeutic trial of a drug should be discussed at the outset of treatment, so that the patient does not have unrealistic expectations of an immediate improvement in symptoms. Patients are more likely to experience side effects early in the course of pharmacotherapy than any relief from their disorder. In some cases, medication may even exacerbate some symptoms. Patients should be counseled that a poor initial reaction to medication is not an indicator of the ultimate outcome of treatment. For instance, many patients with panic disorder develop jitteriness or an increase in panic attacks after starting on tricyclic or SSRI treatment. Benzodiazepine agonists are an exception to the rule that clinical onset is delayed. In most cases, their hypnotic and antianxiety effects are evident immediately.
Ongoing use of medication, however, does not provide absolute protection against relapse. Continuation therapy provides clinically and statistically significant protective effects against relapse. The optimal duration of continuation or maintenance therapy is variable and dependent on the clinical history of the patient. Early-onset chronic major depression, for example, has a more severe course and greater comorbidity than late-onset chronic major depression. In addition to early onset, a history of multiple past episodes and severity and length of a current episode would make longer, even indefinite, treatment appropriate.
Frequency of Visits
Until an unequivocal response to treatment occurs, patients should be seen as frequently as circumstances warrant. The frequency of follow-up or monitoring visits is determined by clinical judgment. In severely ill patients, this might mean several times a week. Patients on maintenance therapy, even when stable, need monitoring, but no consensus exists on the frequency of follow-up therapy. Three months is a reasonable interval between visits, but 6 months may be adequate after long-standing treatment.
LABORATORY TESTS AND THERAPEUTIC BLOOD MONITORING
Laboratory testing and therapeutic blood monitoring should be based on clinical circumstances and the drugs being used. For most commonly used psychotropic drugs, routine testing is not required. No currently available laboratory test can confirm the diagnosis of a mental disorder.
Pretreatment tests are routine as part of a workup to establish baseline values and to rule out underlying medical problems that may be causing the psychiatric symptoms or that might complicate treatment with drugs. Results of recently performed tests should be obtained. With agents known to cause cardiac conduction changes, a pretreatment electrocardiogram (ECG) should be obtained before initiating treatment. With lithium and clozapine, the possibility of serious changes in thyroid, renal, hepatic, or hematological functions requires pretreatment and ongoing monitoring with appropriate laboratory tests.
As a result of both anecdotal and research findings of sometimes severe glucose dysregulation during treatment primarily with SDAs, the FDA has suggested that patients being treated with any atypical antipsychotic be monitored for the emergence of diabetes.
Certain circumstances present in which it is necessary or useful to use plasma concentrations to monitor a patient’s condition. These include the monitoring of drugs with narrow therapeutic indexes, such as lithium; drugs with a therapeutic window, the optimal dose range for a therapeutic response; drug combinations that can lead to interactions that raise drug concentrations of medications or their metabolites, which can cause toxicity; unexplained toxicity at normal therapeutic doses; and failure to respond in a patient who may be noncompliant. A clinician should have no reservations about requesting random urine toxicological tests in a patient who abuse substances.
TREATMENT OUTCOMES
The goal of psychotropic treatment is to eliminate all manifestations of a disorder, thus enabling the patient to regain the ability to function as well and to enjoy life as fully as before he or she became ill. This degree of improvement to below the syndromal threshold is defined as remission.
Response and Remission
Remission is the preferred outcome of treatment, not only because of the immediate impact on functioning and state of mind, but also because emerging evidence suggests that patients in remission are less likely to experience relapse and recurrence of their disorder.
Patients who improve but do not experience a full resolution are considered to be responders. They may exhibit significant improvement but continue to experience symptoms. In depression studies, response is usually defined as a 50 percent or greater decrease from baseline on a standard rating scale, such as the Hamilton Depression (HAM-D) Scale or the Montgomery-Asberg Depression Rating Scale (MADRS). Remission is defined as an absolute score of 7 or less on the HAM-D or 10 or less on the MADRS. Expectations about the likely degree of improvement should be based on what is known about the responsiveness of specific disorders to medication therapy. Obsessive-compulsive disorder (OCD) and schizophrenia, for example, are more likely to be associated with residual manifestations of illness than major depression or panic disorder. The probability of full remission from OCD with SSRI treatment alone over a 2-year period is less than 12 percent, and the probability of partial remission is approximately 47 percent.
Treatment Failure
The initial treatment plan should anticipate the possibility that the medication may be ineffective. A next-step strategy should be in place at the initiation of treatment. Repeated drug failures should prompt reassessment of the patient. First, was the original diagnosis correct? In answering this question, the clinician should include the possibility of an undiagnosed medical condition or recreational drug use as the cause of the psychiatric symptoms.
Second, are the observed symptoms related to the original disorder, or are they actually adverse effects of the drug treatment? Some antipsychotic drugs, for example, can produce akinesia, which resembles psychotic withdrawal, or akathisia and neuroleptic malignant syndrome, which resemble increased psychotic agitation. Long-term use of SSRIs can produce emotional blunting, which can mimic depression.
Intolerance of side effects may be the most common reason for treatment failure. Third, was the drug administered at an appropriate dosage for a sufficient length of time? Because absorption and metabolism of drugs can vary greatly in patients, the clinician may need to measure plasma levels of a drug to ensure a sufficient dose of the drug.
Fourth, did a pharmacokinetic or pharmacodynamic interaction with another drug that the patient was taking reduce the efficacy of the newly prescribed drug?
Fifth, did the patient take the drug as directed? Drug noncompliance is a common clinical problem that arises as a result of complicated drug regimens (more than one drug in more than one daily dosage), adverse effects (especially if unnoticed by the clinician), and poor patient education about the drug treatment plan. Patients may discontinue medication when they recover, thinking that they are cured and no longer benefiting from the medication.
Treatment Resistance
Some patients fail to respond to repeated trials of medication. No single factor can explain the ineffectiveness of the various interventions in these cases. Strategies in these cases include the use of drug combinations, high-dose therapy, and use of unconventional drugs. Limited evidence is available on the comparative success rates associated with any given strategy.
Tolerance
The development of tolerance is marked by a need, over time, to use increased doses of a drug for it to maintain a clinical effect. This decreased responsiveness to a drug occurs after repeated doses. Tolerance also describes decreased sensitivity to adverse effects of the drug, such as nausea. This phenomenon is used as the basis for starting some drugs at subtherapeutic doses, with the plan to adjust the schedule once the patient can tolerate higher doses. Clinical tolerance appears to represent changes in the CNS, such as altered receptor configuration or density. Drugs with similar pharmacological actions often exhibit cross-tolerance.
Sensitization
Clinically manifested as the reverse of tolerance, sensitization is said to occur when sensitivity to a drug effect increases over time. In these cases, the same dose typically produces more pronounced effects as treatment progresses.
Withdrawal
The development of physiological adaptation to a drug, with a subsequent risk of withdrawal symptoms, has been reported for many classes of psychotropic drugs. Technically, withdrawal should be considered a side effect. The probability and severity of these reactions are remote with most drugs and more common with others. As a general rule, the more abruptly a drug is stopped and the shorter its elimination half-life, the more likely it is that clinically significant withdrawal symptoms will occur. When using some short-acting drugs, withdrawal reactions can result from missed doses and during daily intervals between doses. Gradual tapering of medications after prolonged use is recommended whenever possible. Although this reduces the risk of withdrawal reactions, it does not ensure they will not occur. So-called sedative hypnotics and opiates are the agents most often associated with mentally and physically distressing discontinuation reactions. In some cases, such as barbiturate use, withdrawal can be fatal.
Marked differences are found among agents, even within a given class, with respect to the probability and severity of discontinuation effects. For example, among the benzodiazepines, alprazolam and triazolam (Halcion) commonly produce more immediate and intense withdrawal symptoms than other compounds. Among the SSRIs, there is a well-described withdrawal syndrome that appears to be more frequent and severe with paroxetine (Paxil). It can, however, occur with any SSRI. Even fluoxetine can be associated with discontinuation symptoms, but the symptoms may be delayed and attenuated because of the long elimination half-life of its active metabolite. These manifestations are subtle and are delayed for weeks after the last dose. Venlafaxine also produces a severe SSRI-like withdrawal syndrome.
In addition to half-life, many variables can influence the likelihood and degree of discontinuation symptoms. Changes in the rate of drug metabolism, as an example, can play a role. Paroxetine is primarily metabolized by the cytochrome P450 (CYP) 2D6 isoenzyme, however, it is also a potent inhibitor of CYP 2D6. This results in autoinhibition, a dose-dependent inhibition of its own metabolism, with a subsequent increase in plasma concentrations of paroxetine. If the dose of paroxetine is decreased or the drug is stopped, the decline in its plasma concentrations can be steep, causing withdrawal to occur. Withdrawal can occur in rare cases in which the dose of a drug is not decreased, but a second agent, which had been inhibiting its metabolism, was stopped. For example, alprazolam is metabolized via the CYP 3A3/4 enzyme system. Nefazodone inhibits that enzyme. If a patient taking both agents for several weeks discontinues the nefazodone, it could result in a rapid increase in the rate of alprazolam metabolism and a consequent drop in plasma concentrations.
The development of sustained-release versions of drugs, such as alprazolam, paroxetine, and venlafaxine, has not reduced the severity of their withdrawal reactions. The prolonged half-life of those agents results from delayed absorption rather than prolongation of the elimination phase. The frequency of drug dosing is reduced but not the rate of falloff in plasma concentrations.
Poor bioavailability with a generic agent may account for unexpected loss of clinical effect in emergence of withdrawal symptoms. The occurrence of these events soon after refilling a prescription should prompt examination of the new medication. It should be confirmed whether the dispensed medication and dose are both correct. It is difficult to ascertain whether generic medications are truly equivalent, so the possibility exists that differences in potency may underlie adverse changes in clinical status.
Withdrawal symptoms invariably occur hours or days after dose reduction or discontinuation. Symptoms resolve within a few weeks, so the persistence of symptoms argues against withdrawal. Although depletion studies have been shown to provoke rapid return of symptoms, in clinical practice, psychotic and mood symptoms do not usually reappear abruptly after long-term treatment.
COMBINATION OF DRUGS
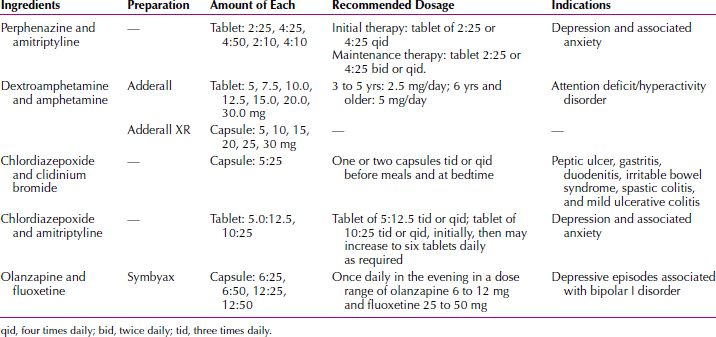
According to the American Psychiatric Association Practice Guidelines for the Treatment of Psychiatric Disorders, “the use of multiple agents should be avoided if possible” in the treatment of psychiatric disorders. Although monotherapy represents the ideal, polypharmacy, the simultaneous use of psychotropic medications, has been commonplace since chlorpromazine was combined with reserpine (Diupres) in the early 1950s. The practice of combining drugs and the merits of various augmentation or combination strategies are routinely discussed in the literature and at scientific meetings. The mean number of simultaneously prescribed medications has increased in recent decades. Among psychiatric inpatients, the mean number of psychotropics prescribed is approximately three. Fixed combinations—drugs that contain more than one active ingredient—have been successfully marketed in the past, and research on new combinations is ongoing. A fluoxetine-olanzapine fixed combination has been approved as a treatment for bipolar disorder. The use of such drugs may increase the patients’ compliance by simplifying the drug regimen. A problem with combination drugs, however, is that the clinician has less flexibility in adjusting the dosage of one of the components; that is, the use of combination drugs can cause two drugs to be administered when only one drug continues to be necessary for therapeutic efficacy (Table 29.1-3).
 Table 29.1-3
Table 29.1-3
Combination Drugs Used in Psychiatry
Sometimes distinctions are made between augmentation and combination therapy. When two psychotropics with the same approved indications are used concurrently, this is termed combination therapy. Adding a drug with another indication is termed augmentation. Augmentation often entails use of a drug that is not primarily considered a psychotropic. For example, in treating depression, it is not common to add thyroid hormone to an approved antidepressant.
Almost all patients with bipolar disorder are taking more than one psychotropic agent. Combination treatment with drugs that treat depression and dopamine receptor antagonist or serotonin-dopamine antagonist has long been held as preferable in patients with psychotic depression. Similarly, SSRIs typically produce partial improvement in patients with OCD, so the addition of a serotonin-dopamine antagonist may be helpful.
Medications also can be combined to counteract side effects, to treat specific symptoms, and as a temporary measure to transition from one drug to another. It is common practice to add a new medication without the discontinuation of a prior drug, particularly when the first drug has provided partial benefit. This can be done as part of a plan to transition from an agent that is not producing a satisfactory response or as an attempt to maintain the patient on combined therapy.
Advantages of combining drugs include building on existing response, which may be less demoralizing, and the possibility that combinations produce new mechanisms that no single agent can provide. One limitation is that noncompliance and adverse effects increase, and the clinician may not be able to determine whether it was the second drug alone or the combination of drugs that resulted in a therapeutic success or a particular adverse effect. Combining drugs can create a broad spectrum effect and also changes the ratio of metabolites.
COMBINED PSYCHOTHERAPY AND PHARMACOTHERAPY
Many psychiatrists believe that patients are best treated with a combination of medication and psychotherapy. Studies have demonstrated that the results of combined therapy are superior to those of either type of therapy alone. When pharmacotherapy and psychotherapy are used together, the approach should be coordinated, integrated, and synergistic. If the psychotherapy and the pharmacotherapy are directed by two separate clinicians, the clinicians must communicate with each other clearly and often.
SPECIAL POPULATIONS
Although every patient brings a unique combination of demographic and clinical variables to the clinical setting, certain patient populations require special consideration. When treating the young, the elderly, those with medical disorders, and women who want to conceive, are pregnant, or are nursing, awareness of risks associated with medication assumes increased importance. Data derived from clinical trials are of limited value in guiding many decisions, because populations in these studies consisted of healthy young adults and, until recently, excluded many women of child-bearing age. Studies of children and adolescents have become more common, so understanding of treatment effects in this population has grown.
Children
Understanding of the safety and efficacy of most psychotropic drugs when used to treat children is based more on clinical experience than on evidence from large clinical trial data. Other than attention-deficit/hyperactivity disorder (ADHD) and OCD, commonly used psychotropic drugs have no labeling for pediatric use, so results from adult studies are extrapolated to children. This is not necessarily appropriate because of developmental differences in pharmacokinetics and pharmacodynamics. Dosing is another special consideration in drug use with children. Although the small volume of distribution suggests the use of lower doses than those used in adults, a child’s higher rate of metabolism suggests that a higher ratio of milligrams of drug to kilograms of body weight should be used. In practice, it is best to begin with a small dose and to increase it until clinical effects are observed. The clinician should not hesitate, however, to use adult dosages in children if these dosages are effective and the adverse effects are acceptable.
The paucity of research data is a legacy of many years in which manufacturers avoided conducting trials in children because of liability concerns, small market share, and, hence, limited profit potential represented by this population. To correct this problem, the FDA Modernization Act (FDAMA) of 1997 provided for special encouragement and incentives to study drugs for pediatric use.
Pregnant and Nursing Women
No definitive assurances exist that any drug is completely without risk during pregnancy and lactation. No psychotropic medication is absolutely contraindicated during pregnancy, although drugs with known risks of birth defects, premature birth, or neonatal complications should be avoided if acceptable alternatives are available.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








