Psychopharmacology of Patients with Behavior Disorders and Epilepsy
Michael R. Trimble
Marco Mula
Introduction
Psychiatric disorders are common in patients with epilepsy, and they encompass the spectrum of conditions from those that are a direct consequence of the epileptogenic activity to others that are simply comorbid.45
Therapy for behavioral disorders still remains unsatisfactory, and many patients with epilepsy receive psychotropic medications (Table 1) not always based on their psychiatric symptoms.81 Effective therapy depends on a correct diagnosis and a combination of psychotropic drug treatments and behavioral interventions when other factors, such as emotional stressors, are present. In addition, antiepileptic drugs (AEDs) are important psychotropic agents with positive and negative psychotropic properties in their own right that should be taken into account when evaluating psychiatric symptoms in patients with epilepsy.
The present chapter focuses on the main problems that a clinician may encounter when treating psychiatric disorders in patients with epilepsy. We examine the effects of AEDs on mood and behavior, and we briefly review main factors that may affect choice of therapy, and patient’s response and compliance, when prescribing antidepressants or antipsychotic drugs. In reviewing these agents, we concentrate specifically on drug interactions and any potential proconvulsive risk they may pose.
Antiepileptic Drugs as Psychotropic Agents
During the last 15 years, the number of AEDs available clinically has nearly trebled,71 thus providing the possibility of providing better-tailored therapy according to patients’ needs but also revealing a wide spectrum of adverse effects.
In some cases, emergent psychiatric symptoms may be side effects of the AED therapy,5,42 the result of interactions between the drug and some biologic vulnerabilities of the patient. Subjects with more severe forms of epilepsy are generally more at risk to develop psychiatric adverse events (PAEs),60,62 whereas specific lesions in the limbic system, such as hippocampal sclerosis, seem to be associated with the liability to develop depressive symptoms.61 The forced normalization phenomenon is well known to be an idiosyncratic reaction to a sudden seizure cessation that may happen with different AEDs in predisposed patients, but whose characteristics are largely unknown.59
On the other hand, we have to take into account the fact that AEDs are extensively used in psychiatric practice for a broad spectrum of psychiatric disorders, especially bipolar disorders, and some are well known to stabilize mood. Since its introduction into the clinical management of epilepsy, carbamazepine has been reported to have psychotropic properties. Over time, several controlled studies have been carried out comparing the effects of carbamazepine in acute mania with placebo, lithium, or neuroleptics.15 These studies have shown that carbamazepine is equivalent to lithium over a period of 8 weeks, and that the time course of the antimanic effect is a little slower than with neuroleptics but equivalent to lithium. This is relevant for those patients who are refractory to lithium and require an alternative to it. Carbamazepine has also been shown to be an effective treatment for the prophylaxis of bipolar disorder, with controlled studies suggesting that patients who are referred to as rapid cyclers (namely, patients with an unstable bipolar disorder with rapid fluctuations of more than four episodes a year), do best on carbamazepine or a combination of carbamazepine and lithium.93 This approach has several advantages over the use of neuroleptics for such conditions, such as the avoidance of tardive extrapyramidal symptoms.
Valproate has been used in manic episodes, depressive episodes, and the maintenance therapy of bipolar disorder.96 The strongest supporting evidence is for it use in acute mania, with somewhat less supporting evidence for the other conditions. Valproate may have an adverse effect on behaviors such as affective lability, aggression, and impulsivity across a range of different clinical contexts but, at the moment, controlled studies are available mainly for bipolar depression.
Among the new AEDs, some of them (e.g., tiagabine) have failed to show any efficacy in primary psychiatric disorders whereas others (e.g., topiramate) may have adjunctive uses, such as weight loss in the management of obesity. The data on the effects of oxcarbazepine on psychiatric disorders are limited and definitely less conclusive than those regarding carbamazepine. However, oxcarbazepine seems to be less effective than lithium but as effective as carbamazepine in acute mania; oxcarbazepine is probably better tolerated than carbamazepine.94 The lack of efficacy of gabapentin in bipolar disorders has emerged from controlled studies have failed to detect such an effect.29
During clinical trials in the development of lamotrigine as an AED, it was observed that it had antidepressant properties. The cumulative results of the studies done so far provide evidence that lamotrigine is effective in the management of the depressed phase in bipolar disorder type II and in the long-term stabilization of mood in patients with rapid-cycling bipolar disorder.36
Among AEDs in development, pregabalin is probably the most interesting molecule. Controlled studies have demonstrated that it is better than placebo in anxiety disorders such as generalized anxiety disorder.69
Table 1 Brief classification of psychotropic drugs | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Thus, data published to present suggest an important role for AEDs in psychiatric disorders. Unfortunately, it is difficult
to extrapolate findings from these studies, performed with psychiatric patients, directly to patients with epilepsy. Obviously, it would be very useful to know whether AEDs have a positive influence on the psychic status of patients with epilepsy beyond their influence on seizure activity. However, there is little scientific evidence for this; most of the studies are uncontrolled and based on quality-of-life parameters rather than on a formal psychiatric evaluation. Because the simultaneous use of an AED as an anticonvulsant and antidepressant or mood stabilizer should be an important option for a rational pharmacotherapy in patients with uncontrolled epilepsy and comorbid psychiatric disorders, the need is great for further studies.
to extrapolate findings from these studies, performed with psychiatric patients, directly to patients with epilepsy. Obviously, it would be very useful to know whether AEDs have a positive influence on the psychic status of patients with epilepsy beyond their influence on seizure activity. However, there is little scientific evidence for this; most of the studies are uncontrolled and based on quality-of-life parameters rather than on a formal psychiatric evaluation. Because the simultaneous use of an AED as an anticonvulsant and antidepressant or mood stabilizer should be an important option for a rational pharmacotherapy in patients with uncontrolled epilepsy and comorbid psychiatric disorders, the need is great for further studies.
During the last few years, lamotrigine is the only AED investigated also as a psychotropic drug in patients with epilepsy. A randomized, placebo-controlled, double-blind, cross-over study of lamotrigine in 81 patients with refractory partial seizures showed some improvement in two subscales (happiness and alertness) in a health-related quality of life model but not on the other four scales specific for self-esteem, mood, anxiety, and depression.82 Two double-blind studies of la-motrigine verus, respectively, carbamazepine27 and valproate16 demonstrated some improvement in quality of life outcomes. An open study40 reported a significant antidepressant effect of lamotrigine in 13 patients with uncontrolled epilepsy and depression. Moreover, in two different studies, we have observed that lamotrigine significantly reduced the occurrence of PAEs during therapy with topiramate60 or levetiracetam.62 All these studies taken together suggest a possible role of lamotrigine as an antidepressant or mood stabilizer in those patients with uncontrolled epilepsy and depressive symptoms.
Pharmacokinetic Interactions Between Antiepileptic Drugs and Psychotropic Drugs
Pharmacokinetic Interactions with Antidepressants
Several factors must be taken into account when predicting the outcome of a potential interaction: Patient-related (sex, age, ethnicity) and drug-related (the presence of active metabolites, the activity and potency at the enzyme site, the therapeutic window).70 The role of the CYP450 enzyme system and glucuronosyltransferases (UGTs) in clinical psychopharmacology is increasingly recognized, and many papers have been published about pharmacokinetic interactions between AEDs and psychotropic drugs.55,57,58
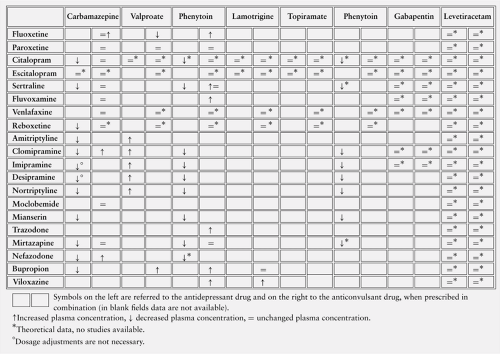
Tricyclic antidepressants (TCAs) such as amitriptyline, clomipramine, and imipramine, are mainly metabolized by CYP1A2, -2D6, and -3A4 (Table 2). Nortriptyline and desipramine are, respectively, the active metabolites of amitriptyline and imipramine and are subsequently metabolized by CYP2D6.80 Moclobemide is primarily metabolized by the CYP2 C subfamily, of which it is probably an inhibitor,28 whereas the atypical antidepressants mianserin and trazodone are metabolized by CYP2D6.11
The selective serotonin reuptake inhibitors (SSRIs) fluoxetine and paroxetine are metabolized by CYP2D6, whereas sertraline, fluvoxamine, and citalopram are respectively metabolized by CYP3A4, -1A2, and -2 C.64 Paroxetine and fluvoxamine are inhibitors of CYP2D674 and -1A2,7 respectively (Table 3). Fluoxetine is a moderate inhibitor of CYP3A4, but, like paroxetine, is a potent inhibitor of CYP2D6. No clinically significant induction-inhibition properties have been demonstrated for sertraline and citalopram.64
Among the new generation of antidepressant drugs, venlafaxine is primarily metabolized by CYP2D6,17 whereas CYP3A4 metabolizes nefazodone and reboxetine.87 Nefazodone is a potent inhibitor of this enzymatic pathway.87
Generally, phenobarbital, carbamazepine, and phenytoin stimulate the metabolism of TCAs, whereas valproate can increase their plasma levels55 (Table 4). An open-label study investigated the effect of valproate on amitriptyline and its active metabolite nortriptyline.97 The mean area under curve (AUC) and the peak plasma concentration, for the sum of nortriptyline and amitriptyline, was 42% and 19% higher, respectively.
In a case series of 13 patients with major depression, the effects of carbamazepine on imipramine and desipramine serum concentrations have been investigated.89 The authors demonstrated that carbamazepine affects not only the metabolism of both drugs but also their protein binding, thus leading to a significant increase in the free fraction.
Table 2 CYP enzymes involved in psychotropic drug metabolism | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Table 3 CYP enzymes inhibited or induced by different psychotropic drugs | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
Data about fluoxetine-carbamazepine interactions are contradictory and are still based on two old studies. The first one is a formal pharmacokinetic study using healthy male volunteers, in which the authors observed a slight increase in carbamazepine AUC levels and a decrease in 10,
11-carbamazepine epoxide AUC.30 The second study is a small series of eight patients with epilepsy who showed no modifications in carbamazepine plasma levels before and after fluoxetine administration.85 These two studies are not comparable mainly because the activity of CYP enzymes is influenced by different factors, namely age, sex (in the first study the authors used only male patients), ethnicity, and so on.
11-carbamazepine epoxide AUC.30 The second study is a small series of eight patients with epilepsy who showed no modifications in carbamazepine plasma levels before and after fluoxetine administration.85 These two studies are not comparable mainly because the activity of CYP enzymes is influenced by different factors, namely age, sex (in the first study the authors used only male patients), ethnicity, and so on.
The inhibition properties of several SSRIs on phenytoin metabolism have been tested in an in vitro study with human liver microsomes.86 The risk for a phenytoin-SSRI interaction seems to be higher with fluoxetine and less likely with the others (paroxetine and sertraline).
Possible kinetic interactions between paroxetine and carbamazepine, valproate, and phenytoin have been investigated in a single-blind, placebo-controlled, cross-over trial.3
Paroxetine caused no change in plasma concentrations and protein binding of the anticonvulsants. Studies of paroxetine plasma concentrations are lacking, but the major enzymatic pathway is a non-inducible enzyme (CYP2D6); therefore, modifications in plasma levels are unlikely when paroxetine is coadministered with AEDs with inducing properties.
Paroxetine caused no change in plasma concentrations and protein binding of the anticonvulsants. Studies of paroxetine plasma concentrations are lacking, but the major enzymatic pathway is a non-inducible enzyme (CYP2D6); therefore, modifications in plasma levels are unlikely when paroxetine is coadministered with AEDs with inducing properties.
Leinonen et al.51 observed an increase in citalopram levels when carbamazepine was substituted with oxcarbazepine in two patients, demonstrating a significant induction effect of carbamazepine on citalopram metabolism. Steinacher et al. confirmed this observation in an open study of six patients, showing that a 4-week treatment with carbamazepine decreased the plasma concentration of S-citalopram and R-citalopram by 27% and 31%, respectively.88
The potential interaction between carbamazepine and fluvoxamine has been evaluated in a small open study of eight patients with epilepsy in steady-state for carbamazepine; no significant changes in carbamazepine and carbamazepine-10,11-epoxide occurred.85 There are no studies of valproate-fluvoxamine interactions.
In the literature, two studies have investigated a possible effect of sertraline on phenytoin and carbamazepine metabolism. A double-blind, randomized, placebo-controlled study with 30 healthy volunteers showed no modifications in phenyt-oin pharmacokinetics.77 The same authors, in a double-blind, randomized, placebo-controlled study on 14 healthy volunteers, observed no significant effects of sertraline on carbamazepine metabolism.78 Conversely, Pihlsgard and Eliasson clearly showed that phenytoin and carbamazepine significantly reduced sertraline plasma concentrations.72 Bonate et al.6 demonstrated no drug interaction between clonazepam and sertraline in a randomized, double-blind, placebo-controlled, cross-over study with 13 subjects.
No clinical studies are available about potential interactions between venlafaxine and AEDs, whereas a randomized, cross-over study with 18 male subjects showed no pharmacokinetic interactions between venlafaxine and diazepam.92
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree