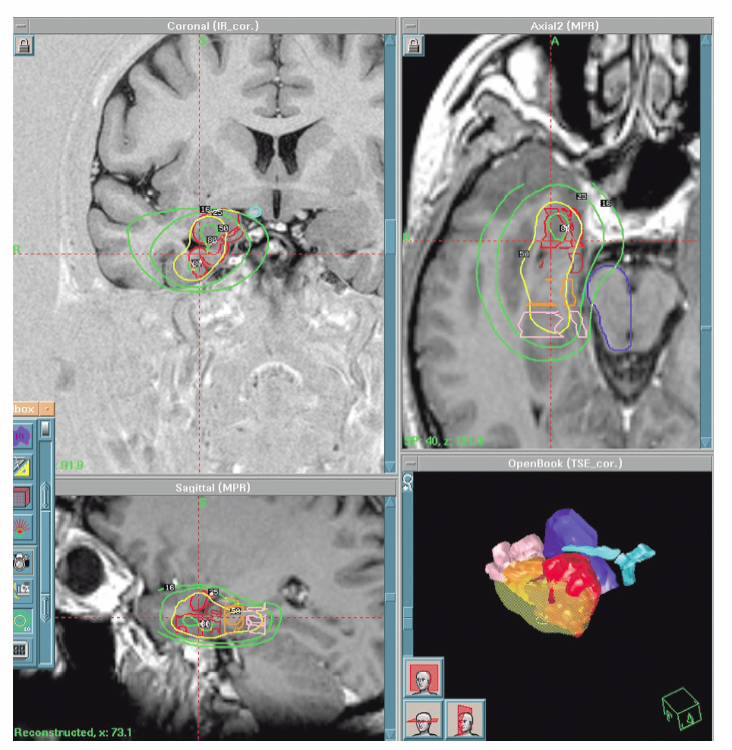
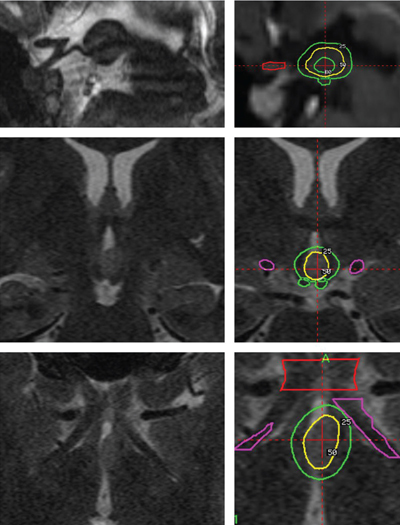
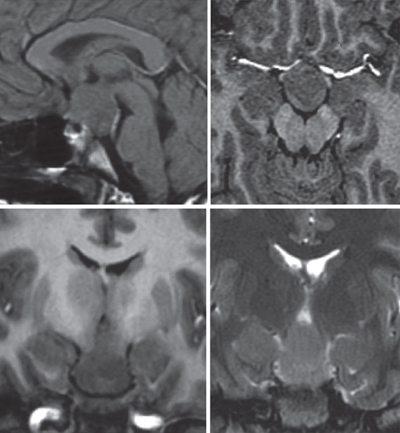
20 Radiosurgery There is growing interest in the use of radiosurgery for treating medically intractable epilepsy. Our local clinical experience (122 patients), accumulated over the past 13 years, mainly includes treatment of temporal lobe epilepsy (56 patients), including 49 with pure mesial temporal lobe epilepsy (MTLE), 55 hypothalamic hamartomas (HHs), two callosotomies, and 11 other kind of epilepsies. The review of our cases, as well as other clinical and experimental data, suggests that the use of radiosurgery is beneficial only to those patients in whom a strict preoperative definition of the extent of the epileptogenic zone (or network) has been achieved, and where strict rules of dose planning have been followed. As soon as these principles are not observed, the risk of treatment failure and or side effects increases dramatically. Long-term outcome data are presently available for MTLE but not yet for other epilepsies. There are convincing arguments for investigating the potential role of radiosurgery for medically intractable seizures. Among 6102 Gamma Knife (GK) surgery procedures accomplished in our neurosurgical unit in a 14-year period (between July 1992 and December 2006), only 122 were performed in patients referred for epilepsy surgery (nine patients/year). The strategy of our treating team is to define the niche of patients in whom the safety/efficacy ratio makes radiosurgery advantageous or at least comparable to craniotomy. Radiosurgery represents a small subset (15%) of patients in our overall epilepsy surgery series. Lars Leksell conceived GK radiosurgery as a tool for functional neurosurgery.1, 2 Accordingly, he used GK in movement disorders, trigeminal neuralgia, and other pain syndromes, but not for epilepsy surgery.3 The first radiosurgical treatments for epilepsy surgery were performed by Talairach in the fifties.4 Unlike Leksell, he had specific expertise in epilepsy surgery and led one of the first large comprehensive programs for epilepsy surgery. As early as 1974, he reported on the use of radioactive Yttrium implants in patients with MTLE without space-occupying lesions, and he showed a high rate of seizure control in patients with epilepsies confined to the mesial structures of the temporal lobe.4 In 1980, Elomaa,5 promoted the idea of the use of focal irradiation for the treatment of temporal lobe epilepsy, based on the preliminary reports of Baudouin and Von Wieser.6,7 Furthermore, clinical experience of the use of GK- and Linac-based radiosurgery in AVMs and cortico-subcortical tumors (mostly metastases and low-grade glial tumors) revealed an anticonvulsive effect of radiosurgery in absence of tissue necrosis.8–10 A series of experimental studies in small animals confirmed this effect11,1 2 and emphasized its relationship to the dose delivered.13–16 Barcia Salorio et al, and later Lindquist et al, reported small and heterogenous groups of patients treated with the purpose of seizure cessation; their results, however, were poor.17 – 20 Unfortunately, these results were never published in peer-reviewed articles. The Department of Stereotactic and Functional Surgery at Timone University Hospital in Marseille is a major referral center for epilepsy surgery and radiosurgery. This expertise has facilitated the investigation and development of a potential role for GK radiosurgery in the treatment of intractable epilepsy. Since 1993, we have performed 122 cases of epilepsy surgery using GK radiosurgery. The majority of these patients presented with MTLE (56 patients) or HH (55 patients). The rest of the patients suffered from severe epilepsy associated with small benign lesions (11 patients), for which an epileptic zone was considered to be confined to the surrounding cortex.21 In HH, GK radiosurgery offers very low morbidity, with similar efficacy when compared with microsurgical alternatives.22,23 This has led us to consider radiosurgery systematically as the first-line treatment in patients with small HH of type I, II, III, and possibly type IV.23 In MTLE, on the other hand, in spite of a good short-and middle-term safety-efficacy ratio,24–26 the use of GK is still regarded as investigational,25 given the well-establishedlong-term safety and efficacy of the microsurgical resection in the temporal lobe. Since 1994, we have promoted the idea that seizure cessation may be generated by a specific neuromodulatory effect of radiosurgery, without induction of a significant amount of histological necrosis.27–32 The selection of the appropriate technical parameters (dose, volume target, etc) allowing us to accurately obtain the desired functional effect without histological damage, remains an important challenge (Fig. 20.1). Fig. 20.1 Gamma Knife radiosurgery for a right MTLE. The dose is 24 Gy at the 50% isodose line (yellow). Doses to the brainstem are <12 Gy (25%), and the dose to the optic chiasm is <8 Gy (16%). Note the sparing of a large part of the amygdaloid complex and hippocampal head. Complete seizure cessation occurred 12 months after radiosurgery with no complication and no (even transient) side effects. HHs may be asymptomatic, associated with precocious puberty or with neurological disorders (including epilepsy, behavior disturbances, and cognitive impairment), or both. Usually, the seizures begin early in life and are often particularly drug-resistant from the outset. The natural history is unfavorable in the majority of the patients because of behavioral symptoms (particularly aggressive behavior) and mental decline, which occur as a direct effect of the seizures.33 Interestingly, in our experience, the reversal of these behavioral symptoms after radiosurgery seems to begin even before complete cessation of the seizures and seems to be correlated to the improvement in background EEG activity. It is the authors’ speculation that these continuous discharges lead to the disorganization of several systems, including the limbic system, and that their disappearanceaccounts for the improvement seen in attention, memory, cognitive performance, and impulsive behavior, etc. In these cases, radiosurgery’s role in the reversal of the behavioral symptoms may be as or more important than its effect on decreasing seizures. Consequently, we consider that it is essential to operate on these young patients as early as possible, whatever the surgical approach considered (resection or radiosurgery). The intrinsic epileptogenicity of HH has been demonstrated34,35 even though the mechanisms of the epilepsy associated with HH are still debatable. The boundary of the target zone of treatment is that of the lesion visualized on magnetic resonance imaging (MRI). This contrasts greatly with cases of MTLE where there is no such clear delineation of an epileptogenic zone on the images used for planning radiosurgical intervention. We retrospectively analyzed radiosurgery in a series of 10 patients collected from centers around the world.22 The excellent safety-efficacy ratio (all improved, 50% cured and no adverse effects except one case of poikilothermia) led us to organize a prospective multicenter trial. Our series of 55 prospectively evaluated patients has been published in a preliminary report.23 The number of patients operated by GK more than 3 years ago was 31, with a satisfactory follow-up being available for 27 patients. The preoperative cognitive deficits, behavioral disturbances, and investigated relationship of seizure severity and anatomical type to cognitive abilities were characterized.36,37 The goal of the preoperative workup was to adequately select the candidates for inclusion and to evaluate the baseline neurological and endocrinological functions. All radiosurgical procedures were performed using the Leksell 201-source Cobalt 60 Gamma Knife (Elekta Instrument, Stockholm, Sweden). Consistently we developed multi-isocentric complex dose planning of high conformity and selectivity (Fig. 20.2). We used low peripheral doses to take into account the close relationship with the optic pathways and the hypothalamus (median 17 Gy; range 13 to 26 Gy). The lesions treated are generally small (median 9.5 mm; range 5 to 26 mm, Fig. 20.2). We pay special attention to the dose delivered to the mamillary body and to the fornix, and we always try to tailor the dose plan for each patient, based on the use of a single run of shots with the 4-mm collimator. Patients were evaluated with respect to seizures, cognition, behavior, and endocrine status 6, 12, 18, 24, and 36 months after radiosurgery and then every year. Among those, 10 are seizure free (37%), six are very much improved (22.2%) with a significant seizure reduction (usually only rare residual gelastic seizures) and with a dramatic behavioral and cognitive improvement. Overall an excellent result has been obtained in 60% of patients. Our strategy is to offer the patient and the family are offered a second radiosurgery in case of partial benefit when the lesion is anatomically small and well defined. Five patients (18.5%) with small hamartomas were only modestly improved and are being considered for two sessions of radiosurgery. Two have until now reported no significant improvement. A microsurgical approach has been performed in four patients (14.8%) with quite large HHs and poor efficacy of radiosurgery. After this microsurgical approach, two are cured and two are failures. The radiosurgical treatment has been performed twice in nine patients. The improvement was dramatic, in nine of these patients. All the patients with paroxystic aggressivity improved substantially. Increased alertness, elevated mood, and greater speech production were observed in some patients characterized by excessive behavioral inhibition. A positive effect on sleep was frequently reported by the parents, mainly in the younger patients. Finally, dramatic developmental acceleration was observed in three young patients. Topological classification of the lesion based on good high-resolution MRI is a key feature in the decision-making process.23 Previous classification has been based on anatomical38–40 or surgical41 considerations. These classifications do not describe the large diversity of these lesions and their therapeutic consequences. As underlined by Palmini and coworkers, the exact location of the lesion in relation to the interpeduncular fossa and the walls of the third ventricle correlates with the extent of excision, seizure control, and complication rate.42 On this basis, we classify the HH according to their topology based on our original classification.23,43 In our experience this classification correlates with the clinical semiology and severity and is especially critical for surgical strategy selection. Type I HHs (small HH located inside the hypothalamus extending more or less into the third ventricle) are certainly the best candidates for GK surgery. In this population the morbidity of microsurgical removal is likely to be potentially high. In type II (when the lesion is small and mainly in the third ventricle) radiosurgery is certainly a safer alternative. Even though the endoscopic and transcallosal interforniceal approach has been well described (see Chapter 9), the risks of short-term memory worsening, endocrinological disturbance (hyperphagia with obesity, low tyroxine, sodium metabolism disturbance), and thalamic or thalamocapsular infarcts have been reported even in the hands of highly skilled and experienced neurosurgeons. In exceptional cases of recurrent status epilepticus we recommend open surgery through either a transcallosal interforniceal approach or an endoscopic approach (depending on the width of the third ventricle). If the lesion is small and the third ventricle large, an endoscopic approach is chosen. Fig. 20.2 Typical good indication of radiosurgery in hypothalamic hamartoma type II according to our original classification (ref). The dose at the margin was 17 Gy.23,43 The volume of the target is 268,2 mm3. The average number of seizures prior to radiosurgery was 300 per month. Five months after radiosurgery seizures have disappeared completely. In type III (lesion located essentially in the floor) with the extremely close relationship between the mammilary body, the fornix, and the lesion, we prefer GK surgery. We speculate that sessile HHs have always more or less of an “extension” into the hypothalamus close to the mammilary body. Thus when a lesion is classified as a type II, that means that the lesion appears on the MRI as being located in the third ventricle but is likely to have a “root” in the hypothalamus. The same assumption is made for type III. In type IV (the lesion sessile in the cistern) a disconnection can be discussed (pterional approach with or without orbitozygomatic osteotomy). However, if the lesion is small, GK surgery can be recommended due to its safety and to its ability to simultaneously treat the small associated part of the lesion in the hypothalamus itself, frequently visible on the high-resolution MRI. In Delalande’s experience only two patients among 14 are seizure free after a single disconnection through a pterional approach.41 Consequently, we only recommend this approach in cases of lesions too large for GK surgery as a first step of a staged approach. In most circumstances, the patient is improved but not seizure free after the first surgical step, and GKS is organized at 3 months as a second step in the treatment. Type V HHs (pediculate) are rarely epileptic and can be easily cured by radiosurgery or disconnection through a pterional approach. In cases of severe epilepsy the second therapeutic modality will result in a more rapid cessation of seizures. However, a distant extension of the HH in the hypothalamus close to the mammillary bodies must be cautiously searched for on high-resolution MRI, and its discovery will eventually lead to the recommendation of GK surgery, allowing for treatment of both parts of the lesion, especially in cases in which the cisternal component is small. Type VI (giant) are not good candidates for up-front radiosurgery, and in nearly all the cases, a combination of several therapeutic modalities should be utilized (Fig. 20.3). Even if GK surgery does not seem to be suitable when the lesion is large, “radiosurgical” disconnection can been be envisaged. Radiosurgically targetting only the superior part in the hypothalamus and/or the third ventricle leaving untreated all of the lesion lower than the floor has been uniformlydisappointing. In our opinion, this strategy in children may result in a loss of precious developmental time in which a child could be treated effectively. Consequently, we do not advocate such a strategy. When microsurgical resection has left a small remnant in the third ventricle and the patient is not seizure free, we recommend GK surgery. Fig. 20.3 Typical good indication for a combined approach. Our proposal in this case is a disconnection by a pterional approach and then a radiosurgery on the residual. Two major questions remain. First, we know that complete treatment or resection of the lesion is not always necessary for control,44–46 but we do not know how to predict in an individual patient the amount (and mapping) of the HH that must be treated to obtain a complete antiepileptic effect. Second, we know that these patients frequently present with an electroclinical semiology suggesting involvement of the temporal or frontal lobe and which can mimic a secondary epileptogenesis phenomenon.35,47 In our experience, some of these patients can be completely cured by the isolated treatment of the HH, whereas in others, a partial result is obtained, with residual seizures despite a significant overall psychiatric and cognitive improvement. In this second group, it is tempting to propose that such a secondary epileptogenic area accounts for the partial failure. Our initial results indicate that GK surgery is as effective as microsurgical resection with reduced morbidity. GK surgery also avoids the vascular risk related to radiofrequency lesioning or stimulation. The disadvantage of radiosurgery is its delayed action. Longer follow-up is mandatory for proper evaluation of the role of GK surgery. Results are faster and more complete in patients with smaller lesions inside the third ventricle (stage II). The early effect on subclinical EEG discharges appears to play a major role in the dramatic benefit to sleep quality, behavior, and cognitive-developmental improvement. GK surgery can safely lead to the reversal of the epileptic encephalopathy. Due to the poor clinical prognosis of a majority of patients with HH and the invasiveness of microsurgical resection, GK can be now be considered a first-line intervention for small- to middle-sized HH associated with epilepsy, as it can lead to dramatic improvements in these unfortunate young patients. The role of secondary epileptogenesis or of widespread cortical dysgenesis in these patients needs to be further evaluated and understood, to optimize patient selection and define the best treatment strategy. The first GK surgery operations for MTLE were performed in Marseille in March 1993. As far as no similar experience was available at that time in the literature, we were obliged to base our technical choices on the experience of radiosurgery for other pathological conditions. Four patients were treatedwith different technical strategies (dose, volume, target definition). The delayed, huge radiological changes observed some months after radiosurgery30 led us to stop such treatment and follow these first four patients. Due to the clinical safety of the procedure in these patients and the gradual disappearance of the acute changes shown on the MR after some months, we treated several new series of patients under strict prospective controlled trial conditions (with ethical committee approval). The treatment for the following 17 patients was based upon that of the first patient who had a successful outcome (as opposed to the three others who had partial or no effect). This “classic planning” (Fig. 20.1) was based on the use of two 18-mm shots, covering a volume of around 7 cc at the 50% isodose (24 Gy), and has turned out to produce a high rate of seizure cessation.24,26 To decrease morbidity, the targeting was centered on the parahippocampal cortex and spared a significant part of the amygdaloid complex and hippocampus. The refinement of the GK surgery technique, and the desire to find a dose which would create less transient acute changes shown on the MR, led us to reduce the dose from 24 Gy to 20 Gy, and 18 Gy at the margin. However, this brought about a significant decrease in the rate of seizure cessation. We have recently reviewed the long-term follow-up of our first 15 patients operated by GK surgery for MTLE at the state of the art (24 Gy). The mean follow-up was 8 years, and at the last follow-up, 73% were seizure free. These long-term results compare favorably with open surgery. No permanent neurological deficit was reported out of a visual field deficit in nine patients.48 The timetable of events after radiosurgery and the follow-up is quite standardized. Patients are informed that delayed efficacy of radiosurgery is its main drawback. Typically, the frequency of the seizures is not modified significantly for the first few months. Thereafter, there is a rapid and dramatic increase in auras for some days or weeks, and then the seizures disappear. Usually the peak in seizure cessation is observed around the eighth to 18th month with a clear variability in the delay in onset. In one patient, this occurred 26 months after GK radiosurgery. We usually consider a delay of 2 years as a minimum for postradiosurgery follow-up. In the absence of initial radiological changes or clinical benefit, the recommendation is to wait for the onset of the MRI changes and their subsequent disappearance. All our patients had the same pattern of MR changes whatever marginal dose (18 to 24 Gy) and volume of treatment (5 to 8.5 cc) were used. However, the degree of these changes and their delay of onset varied according to the dose delivered to the margin, the volume treated, and the individual patient. To allow an optimal evaluation, we recommend that subsequent microsurgery not be considered before the third year after radiosurgery. Similarly, we believe that a patient who undergoes a craniotomy before the onset of the MR changes has occurred cannot be assumed to have failed radiosurgical treatment. Before considering any further surgery, the reason for the treatment failure needs to be addressed. After reviewing files of patients treated for MTLE with radiosurgery, it was sometimes possible to identify likely causes of failure, such as: Our current strategy of treatment is based on our first series of MTLE patients who were strictly selected and treated systematically with a very simple but very reproducible dose-planning strategy.26,32 The identification of putative improvements in the methodology requires a systematic analysis of the influence of the technical data from our experience and from the literature on the outcome of those patients. The first targets used in functional GK radiosurgery (capsulotomy, thalamotomy of ventral intermedius (VIM) or the centromedianum, pallidotomy) were treated using high doses (300 to 150 Gy) delivered in very small volumes (3 to 5 mm in diameter).3 The goal was to ablate a predefined, very small anatomical structure with stereotactic precision. Significant variability in the delay and amplitude of the MR changes has been reported with defined doses.52,53 Barcia Salorio et al have documented a small and heterogeneous group of patients treated with different types of radiosurgical techniques and variable patient-to-patient dosimetry.54 Some of these patients had no expanding lesion and were treated with very large volumes and very low doses (around 10 Gy). Based on this experience, several teams have suggested that very low doses, as low as 10 to 20 Gy at the margin, should be as effective as the 24 Gy protocol (at the margin) that we used for our first series of patients with MTLE.26 A careful review of the last Congress proceeding of Barcia Salorio et al pools patient data on the margin dose, and the volume and topography of the epileptogenic zone. Moreover, among the 11 patients reported, the real rate of seizure cessation is apparently only 36% (4/11), which is much lower than what we would expect with resection in MTLE.2 In a heterogeneous group of 176 patients, Yang et al confirmed that only a very low rate of seizure control is achieved when low doses (from 9 to 13 Gy at the margin) are used.51 The experience of the radiosurgical treatment of HH indicates that 18 Gy at the margin appears to be a threshold in terms of probability of seizure cessation.22 In this group of patients (36 cases), only one showed MR changes. Themajority of the AVM case patients with worsening seizures were treated with a range of doses between 15 and 18 Gy. Similarly, poor results have been reported by Cmelak et al in one case patient with MTLE treated with Linac-based radiosurgery, with 15 Gy at the 60% isodose line, who underwent surgical resection 1 year later. In this case, the authors first observed a slight improvement followed by an obvious worsening.50 A desescalation study has allowed us to demonstrate poorer results in patients receiving doses of 18 or 20 Gy at the margin as compared with 24 Gy.55,56 Due to the rate of seizure cessation that is achievable by conventional resection, a radiosurgical strategy associated with a much lower rate of seizure cessation appears unacceptable. Fractionated stereotactically guided radiotherapy has been demonstrated to fail systematically in controlling seizures. Among 12 patients treated by Grabenbauer et al, none have achieved seizure cessation; only seizure reduction was obtained in this series.57,58 Experimental studies on small animals have demonstrated the antiepileptic effect of radiosurgery,13,15,59 the dose dependence of this effect,13–15,60 and the possibility of obtaining clear antiepileptic effect without macroscopic necrosis using certain doses.14 Although the rat model of epilepsy is not an ideal model of human MTLE, it is intriguing to notice that similar to our clinical experience in humans, a similar maximum dose range of 40 to 50 Gy is currently the dose range that provides the optimal safety-efficacy ratio. When the target is a lesion that is precisely defined radiologically, the question of the selection of the marginal dose can be quite easily addressed by correlating safety-efficacy individual outcome to the marginal dose. This can be refined based upon stratification according to volume, location, age, etc. However, in patients presenting with MTLE, this process is invalid for two reasons. First, there is no consensus regarding the requirement for the extent of mesial temporal lobe resection. Second, the concept of MTLE syndrome with a stable extent of the epileptogenic zone and surgical target is increasingly a topic of debate.61,62 The volume (in association with marginal dose) is well known to be a major determinant of the tissue effect, as shown in integrated risk/dose volume formulae.63 In the first series of patients that we treated, this marginal isodose volume (or prescription isodose volume) was ~7 cc (range 5 to 8,5 cc). An attempt to correlate dose/volume and the effect on seizures and on the MR changes (as evaluated by volume of the contrast enhancement ring, extent of the high T2 signal, and the importance of the mass effect) has been published.55 In this study, we found, not surprisingly, that the higher the dose and the volume, the higher the risk of having more severe MR changes, but also the higher the chance of achieving seizure cessation. However, these data may have limited value. Hence, more precise identification of those structures of the mesial temporal lobe that need to be “covered” by the radiosurgical treatment may allow more selective, but just as efficacious, dose-planning strategies, in spite of smaller prescription isodose volumes. There is growing evidence to support the organization of the epileptogenic zone in networks, meaning that several different and possibly distant structures are discharging simultaneously at the onset of the electroclinical seizure. This kind of organization explains why the risk of failure is so high when a simple topectomy (without preoperative investigations) is performed in severe drug-resistant epilepsies associated with a benign lesion.64 This has been also reported in MTLE.61,62 Certain nuclei of the amygdaloid complex of the head, body, tail of the hippocampus, of the perirhinal, entorhinal (EC), and parahippocampal cortices may be associated with genesis of the seizures. The role of the EC cortex in epilepsy is supported by experimental studies in animals.65,66 The EC is considered to be the amplifier of the “amygdalohippocampal epileptic system.” The pattern of the associated structures, including that of the structure playing the lead role, can vary significantly from one patient to another.61,62 There is a subgroup of patients who have clonic discharges and the involvement of the EC, amygdale, and head of the hippocampus, with a clear lead role of the EC. Wieser et al have analyzed the postoperative MR images of patients operated by Yasargil (amygdalohippocampectomy) and was able to correlate the quality of the resection of each substructure of the mesial temporal lobe area and the outcome with respect to seizures.67 Only the quality of the removal of the anterior parahippocampal cortex was correlated strongly with a higher chance of seizure cessation.67 We tried to perform a similar study in patients treated with GK radiosurgery.55 We defined and manually drew the limits of subregions on the stereotactic images of all these patients. The amygdala, the head, the body, and the tail of the hippocampus were first delineated. The white matter, the parahippocampal cortex, and the cortex of the anterior wall of the collateral fissure were then separately drawn and divided into four sectors in the rostrocaudal axis, corresponding to the amygdala, the head, the body, and the tail of the hippocampus.55 Whang et al treated patients with epilepsy associated with slowly growing lesions and observed seizure cessation in only 38% (12/31) of the patients68 This kind of observation emphasizes the importance of preoperative definition of the extent of the epileptic zone and of its relationship with the lesion.64,69 In our institution, the philosophy is to adapt the investigations for each individual case. In some patients, the electroclinical data, the structural and functional imaging, and the neuropsychological examination are sufficiently concordant for surgery of the temporal lobe to be proposed without depth electrode recording. In other cases, the level of evidence for MTLE is judged insufficient,and a stereoelectroencephalographic (SEEG) study is performed. The strategy of SEEG implantation is based on the primary hypothesis (mesial epileptogenic zone) and alternative hypotheses (early involvement of the temporal pole, lateral cortex, basal cortex, insular cortex, or other cortical areas). The goal of these studies is to record the patient’s habitual seizures, to establish the temporospatial pattern of involvement of the cortical structures during these seizures. Clearly, in these patients, the high resolution of depth electrode recording allows fine tailoring of surgical resection, according to the precise temporospatial course of the seizures. The main limitation of radiosurgery is that of size of the target (prescription isodose volume). The radiosurgical treatment of MTLE is certainly the most selective surgical therapy for this group of patients. The requirements for precision and accuracy in the definition of the epileptogenic zone is consequently higher. Furthermore, if depth electrode investigation enables demonstration of a particular subtype of MTLE, this can lead to tailoring of the treatment volume and frequently allows this to be reduced. The risk of long-term complications must always be cautiously scrutinized in functional neurosurgery. Radiotherapy is most frequently used in the brain for short-term life-threatening pathologies. The use of radiotherapy in young patients with benign disease such as pituitary adenomas or craniopharyngiomas has been associated with a significant rate of cognitive decline70,71 and tumor genesis72 including some carcinogenesis.73 If the risk of radiation-induced tumor was similar with radiosurgery, we should have by now already observed numerous cases. However, such reported cases74–76 are extremely rare and frequently fail to meet the classic criteria by which tumors are deemed to be “radiation-induced.”77 If this risk exists, it is likely to be around 1/10 000, which is far lower than the mortality risk associated with temporal lobectomy.78–82 Epilepsy is a life-threatening condition. The risk of sudden unexplained death in epileptic patients (SUDEP) is higher than in the general population.83,84 This risk is higher in patients treated with more than two antiepileptic drugs and IQ lower than 70 (as independent factors). Because seizure cessation after surgery reduces the mortality risk to that of the general population,84 microsurgical resection of the epileptogenic zone may confer a benefit in terms of the possibility of immediate seizure cessation and therefore reduced mortality risk, as compared with the more delayed benefits of radiosurgical treatment. Our patients are systematically informed about this disadvantage of radiosurgery. We still consider the use of radiosurgery for MTLE to be investigational. The demonstrated advantages of radiosurgery are the comfort of the procedure, the absence of general anesthesia, the absence of surgical complications and mortality, the very short hospital stay, and the immediate return to the previous level of functioning and employment. Potential sparing of memory function is still a matter of debate and needs to be established using comparative studies. There is also a requirement for further demonstration of long-term efficacy and safety of radiosurgery. Worldwide, microsurgical craniotomies for MTLE are proving to be very satisfactory due to the rarity of surgical complications and a high rate of seizure freedom. In our experience, the most important selection parameters are the demonstration of the purely mesial location of the epileptogenic zone, as well as a clear understanding by the patient of the advantages, disadvantages, and limitations. One other very good indication in our experience is that of patients with proven MTLE but previous failure of open surgery, supposedly due to insufficient extent (usually posteriorly) of resection. The best candidates are young patients, with middle severity epilepsy (working, socially well adapted), a high level of functioning (able to understand well the limits and constraints of radiosurgery), a quite high risk of memory deficit with open surgery (MTLE on the dominant side with little hippocampal atrophy, small deficits in verbal memory preoperatively), and potentially huge social and professional consequences in case of a postoperative memory deficit. The field of epilepsy surgery is a new and promising one for radiosurgery. However, determination of the extent of the epileptogenic zone requires specific expertise, which is crucial to achieve a reasonable rate of seizure cessation. In addition, the huge impact of fine technical detail on the efficacy and eventual toxicity of the procedure means that, at present, its use for these indications remains under investigation, and further prospective work is necessary. 1. Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand 1951;102:316–319 2. Leksell L. Sterotaxic radiosurgery in trigeminal neuralgia. Acta Chir Scand 1971;137:311–314 6. Baudouin M, Stuhl L, Perrard A. Un cas d’épilepsie focale traité par la radiothérapie. Rev Neurol 1951;84:60–63 28. Régis J, Levivier M. M H: Radiosurgery for intractable epilepsy. Tech Neurosurg 2003;9:191–203 48. Bartolomei F, Hayashi M, Tamura M, Rey M, Fischer C, Chauvel P, Regis J. Long term efficacy of Gamma Knife radiosurgery in mesial temporal lobe epilepsy. Neurology, 2008;70:1658–1663
Rationale
Hypothalamic Hamartomas
Effect of Gamma Knife Surgery on Behavior and Cognitive Functions
Topological Classification
Limits and Strengths of Radiosurgery in Hypothalamic Hamartoma
Mesial Temporal Lobe Epilepsy
Technical Questions
The Dose Issue
Target Definition
Patient Selection
Potential Concerns
Current Indications
Conclusions
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








