Brain metastasis is the most common type of intracranial tumor.1 Each year, the number of brain tumor metastases diagnosed far outnumbers the total number of other intracranial tumors.1 Further complicating the clinical picture is the fact that between 21 and 86% of patients with metastases to the brain either have or will develop multiple lesions.1–5 In the United States alone, more than 100,000 patients develop brain metastases each year.6,7 With the advent of improved cancer treatments for extracranial disease and the proliferation of neuroimaging technology, this over-all incidence of intracranial metastasis is likely to rise.7,8 Traditional treatment options for patients with brain metastases include symptomatic medical management with corticosteroids, surgical resection, and whole brain radiation therapy (WBRT). Unfortunately, in large series, the aforementioned treatments when used as sole therapy have led to median survivals of 3 to 6 months.9,10 The findings of two randomized trials suggested improved survival and functional status with a combination of surgical resection and WBRT as compared with WBRT alone.11,12 Nevertheless, a third and larger randomized trial in which patients received 30 Gy WBRT demonstrated no significant survival benefit associated with the addition of surgical resection to WBRT.13 The development of treatment strategies that exceed historically palliative measures and lengthen patients’ survival has been the focus of much investigation.14–18 In most patients with metastatic brain disease, concomitant active systemic disease is common.19–25 As such, aggressive intervention coupled with a low morbidity rate is desirable. Because brain metastases are frequently easily identifiable as well-demarcated lesions on either contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI), they are commonly amenable to stereotactic radiosurgery.26–31 In this chapter we detail the efficacy of radiosurgery for brain metastases. Patients with brain metastases are evaluated from both a neurological and a radiological standpoint before and after any neurosurgical intervention. Neurological function is typically classified based on the Karnofsky performance status (KPS). The KPS is a measure of the patient’s degree of functional independence.32 The scoring on the KPS is as follows: 100 = normal, no complaints; 90 = minor symptoms but able to carry on normal activity; 80 = some symptoms but able to perform normal activity with effort; 70 = self-care but unable to carry on normal activity; 60 = requires occasional assistance and cares for most personal needs; 50 = requires considerable and frequent assistance; 40 = disabled and requires special care and assistance; 30 = severely disabled and hospitalized; 20 = very ill and requiring active supportive care; 10 = moribund and death imminent; and 0 = dead. Changes from baseline in the KPS have been used as a quality of life measure of treatment outcome in some studies. Although the KPS has been used in clinical neuro-oncology for decades, it has not been subjected to a detailed reliability analysis. A search of the National Library of Medicine’s MEDLINE for the years 1966 through 2004 using the search terms Karnofsky performance status and reproducibility of results produced 30 potential articles of which four evaluated interrater or interinstrument reproducibility.33–36 None used the kappa statistic and therefore cannot be assessed as better than class III evidence. There were 27 articles selected for review that did provide information on the use of the KPS as well as articles focusing specifically on the comparison of KPS with other scales or evaluating the utility of the KPS. Since Karnofsky et al’s37 original publication in 1948, the KPS has been included in nearly every major publication on the clinical aspects of malignant glioma. Clearly, a scale that describes the clinical outcome and impact of various treatments on physical performance of patients is a valuable tool. Hutchinson et al38 examined interobserver variability in the use of the KPS. Two pairs of physicians scored 60 patients on the scale (29 unselected emergency patients and 31 patients undergoing chronic hemodialysis). Agreement was only 34% for one pair and 29% for the other pair. Major sources of observer variability appeared to be the lack of specific definitions relating to the major elements of the scale. The authors proposed that recognition of these difficulties could improve the development and validation of other clinical scales. Although not specifically addressing primary brain tumor patients this early study points out the importance of examining the reproducibility of scales used in clinical medicine. Mackworth et al39 studied 200 brain tumor patients comparing the KPS score with a modification of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire scale (EORTC-QLQ). The results were based on 160 patients with complete data. This study found a moderate correlation between the scales (r = 0.48), but more importantly suggested that the KPS was more difficult to interpret at the higher end of the scale (KPS 90 to 100) where higher cognitive and emotional issues would have a higher impact on quality of life. This suggests that in this range a more detailed scale would be needed to assess changes in functional status. Over the range from 50 to 80 where most clinical decision making for clinical trial entry has been used the KPS was highly useful and very sensitive to age. The authors concluded that an assessment based on this type of modified questionnaire would be of benefit as a supplement to the KPS, particularly in brain tumor survivors whose emotional well-being is often complicated by the overall disease process and treatment. The Schaafsma et al40 report is one of the most direct and comprehensive studies focused on reanalyzing the KPS. This study published in 1994 reexamined the KPS and cross-validated it with a quality-of-life measure developed by the European Organization for Research and Treatment of Cancer Study Group in prospective fashion on 139 patients with lung cancer. This study determined that, although the KPS remains a valid and useful tool, other measures are more likely to give useful data on quality of life. Scales such as the EORTC QLQ-C30 provide a broader picture of patients’ health status because they examine the nonphysical aspects of patient function such as their emotional, social, and cognitive well-being rather than physical function alone. Similarly, Osoba et al41 examined the role of a variety of scales in studying patients with brain cancer in clinical trials including the KPS. Based on their evaluation, there is a moderate correlation of the KPS with scales pertaining to motor dysfunction but the KPS and scales addressing levels of daily activity, such as the Barthel Activities of Daily Living Index41, correlate poorly, if at all, with subscales evaluating issues such as emotional distress, visual disorder, and communication deficit. Other studies have used the KPS to validate new or modified outcome indices or have incorporated the KPS into more comprehensive scales addressing clinical outcome, suggesting that the scale continues to serve a central role in the development of new and more precise outcome instruments. Llobera et al42 used the KPS and the Independence in Activities of Daily Living Index42 in 200 cancer patients during the terminal phase of their illness to validate a new scale, the Hebrew Rehabilitation Center for the Aged Quality of Life Index. Chang et al43 found that the KPS correlated closely with the Edmonton Symptom Assessment Scale (ESAS)43 physical functioning subscales in their group of 240 cancer patients examined in a validation study of the ESAS. Maltoni et al44 used the KPS to assist in validating a new palliative prognostic score in terminally ill cancer patients. In the study described by Osoba et al45 a KPS of >70 was used as the inclusion criteria to assess the effect on quality of life and self-assessment scores of recurrence or progression of malignant glioma in patients with good functioning. Using the EORTC-QLQ-Core 30 Items (QLQ-C30) and the Brain Cancer Module (BCM20)45 the scores for patients who had recurrent high-grade gliomas and a KPS score ≥70 were compared with the reported health-related quality of life scores of patients with other cancers. Their scores were similar to those of patients with metastatic cancers and worse than those of patients with localized cancers. The KPS has also been used as a key portion of other more complex patient assessment scales in patients with malignant disease. As described by Gaspar et al46 the Radiation Treatment Oncology Group used the KPS as a key component in developing their recursive partitioning analysis for metastatic intracranial disease. Tortosa et al’s47 comprehensive analysis of 95 patients with known malignant glioma incorporated the KPS into a broad-based index including age, KPS, imaging enhancement, and tumor proliferation rate as independent predictors of survival. The KPS continues to be a widely used physician-based outcome measure, which can be used to indicate the level of disability present and to measure the outcomes of various therapeutic interventions. However, the KPS appears to be relatively insensitive as a measure of quality of life. The interrater reliability is poor and the score tends to be heavily influenced by the age of the patient.38 The scale is also less sensitive at the upper ranges of functioning.39 Another commonly used classification of patients is the Radiation Therapy Oncology Group (RTOG) classification for patients with brain metastases using recursive partitioning analysis (RPA).46,48,49 The RTOG’s RPA classes provide an objective means of stratifying brain metastases patients and has been demonstrated to have prognostic importance. The three RPA classes are as follows: class I: patients <65 years of age, a KPS ≥70 with well-controlled primary disease and no extracranial metastases; class II: patients with KPS <70; and class III: all other patients. The validity and reliability of the RPA classes was verified using RTOG 91–04, a phase III study of accelerated hyperfractionation versus accelerated fractionation and a RTOG brain metastases dataset.46 Additional outcome measures include patient survival, local tumor control, distant brain control, need for corticosteroid medication, and quality of life. Actuarial control or survival is the probability of local control or survival, respectively, for the time defined. This outcome measure becomes less meaningful as the overall number of patients declines. Local tumor control represents the percentage of patients whose tumors demonstrated either stable or decreased volume on follow-up neuroimaging (i.e., MRI or CT). Median survival is the median time interval that patients live after treatment. This is calculated from the time of a specific intervention or the time of tumor diagnosis. The development of a brain metastasis is a common complication of active systemic cancer. The most common primary tumors that metastasize to the brain are lung, breast, melanoma, renal, and colon cancer. In a summary of multiple autopsy studies, the mean percentage of patients who develop brain metastasis from the four major primary cancers are as follows: 33% with primary lung cancer develop a brain metastasis; 21% with breast; 48% with melanoma; and 11% with renal.1 More rarely, other cancers such as prostate, bladder, ovarian, or sarcoma metastasize intracranially. Based upon 1998 data from the American Cancer Society, ~112,960 to 141,200 cancer patients die with brain metastases each year in the United States.7 This number will probably be found to be much higher with more frequent use of brain imaging studies.6,7 As cancer patients live longer as a result of improved therapy for extracranial disease, the incidence of brain metastasis is likely to increase. The average age for developing a brain metastasis is ~60 years old.50 Lung cancer is the most common source of brain metastasis for men. However, breast cancer is the most common source for women.51 Most types of cancers appear to metastasize to the same degree in men as in women. One exception is melanoma; it appears to metastasize more frequently in men.50–53 The distribution of metastasis locations corresponds approximately to the size and blood flow patterns of the brain. This regional tumor distribution is as follows: 80 to 85% in the cerebral hemispheres, 10 to 15% in the cerebellum, and 3 to 5% in the brain stem.4,50 Autopsy studies show that 60 to 85% of patients who die from cancer have multiple brain metastases.50,51 Clinical signs and symptoms of brain metastasis clearly depend upon the size and location of the neoplasm. Approximately two thirds of all brain metastases are symptomatic at least to some degree.9,54 The symptoms can be broadly classified as either related to elevated intracranial pressure or focal neuronal irritation and destruction. The most common symptoms include headache, cognitive or behavioral disturbances, and focal sensory or motor deficits.9,54 These symptoms tend to be of insidious onset, but intratumoral hemorrhage most often associated with melanoma can lead to a more acute presentation. With the more frequent use of neuroimaging, the detection of small, asymptomatic brain tumors including metastases is increasing.55–57 The time interval between diagnosis of the primary malignancy and a brain metastasis varies substantially, and it appears to be dependent in part upon tumor histology. Patients with lung cancer who go on to develop a brain metastasis tend to do so at a median of 6 to 9 months after diagnosis of the primary malignancy.58,59 Renal brain metastases are typically diagnosed 12 months from detection of the primary malignancy.3,60 Colon, breast, and melanoma brain metastases are usually diagnosed 2 to 3 years following discovery of the primary malignancy.61–63 On the other hand, some patients may present with symptomatic brain metastasis before any primary malignancy has been diagnosed. A thorough evaluation of patients with the suggestion of brain metastasis on imaging studies should include a patient history as well as physical, chest, abdomen, and pelvic CT, and blood tests for tumor markers such as carcinoembryonic antigen, fetal antigen 2, and prostate specific antigen. However, 16 to 35% of patients with brain metastasis at initial presentation will have no identifiable primary malignancy.64–66 Later, a lung primary is often diagnosed in those patients with an initially unknown primary malignancy.67,68 The presence of a brain metastasis is frequently found on neurological examination. We did a systematic review of the English-language medical literature to evaluate the detection of brain metastases by clinical examination using the following computer databases: MEDLINE and pre-MEDLINE, and the National Cancer Institute’s CANCERLIT. (We used these databases to determine all of our reviews reported hereafter. Other useful evidence resources include the Cochrane Library, http://www.cochrane.org/; the National Cancer Institute, http://www.cancer.gov/brain; the American Cancer Society for data on the incidence of cancer, http://www.cancer.org/docroot/home/index.asp; the International Radiosurgery Support Association, for a list of clinical trials and noteworthy treatments for brain tumors, http://www.irsa.org/; and the Radiation Therapy Oncology Group, for a list of clinical trials and noteworthy treatments for brain tumors, http://www.rtog.org/). Because lung cancer is the most frequent source of brain metastases and consequently the most well studied, we focused our attention on the literature for lung cancer. Seventeen studies were evaluated.69–85 Eight studies included information on patients with positive and negative findings on neurological examination providing class I evidence regarding the value of neurological examination in detecting metastases (using CT scanning as the gold standard) (Table 6-1).  6
6 
Radiosurgery for Brain Metastases
Jason P. Sheehan, Douglas Kondziolka, with
Timothy C. Ryken
Patient Assessment
Establishing the Diagnosis
| Reference | Type of Cancer | Patients Included | Results Table | |||
|---|---|---|---|---|---|---|
| Osada et al81 | Non–small cell lung | |||||
| Yokoi et al85 | With and without metastasis Non-small cell lung | No clinical findings | ||||
| Cole et al70 | With and without metastasis Bronchogenic | No clinical findings | ||||
| Habets et al73 | With and without metastasis Small cell lung | With and without metastasis | Imaging + (CT) | |||
| Findings | Yes | No | ||||
| Yes | 3 | 1 | 4 | |||
| No | 0 | 50 | 50 | |||
| 3 | 51 | 54 | ||||
| Sensitivity | 100.00% | |||||
| Specificity | 98.04% | |||||
| PPV | 75.00% | |||||
| NPV | 100.00% | |||||
| LR + | 51 | |||||
| LR − | 0 | |||||
| Kormas et al78 | Without known metastasis Non–small cell lung | No clinical findings | ||||
| Salvatierra et al83 | No known metastasis | With and without metastasis | Imaging + (CT) | |||
| Non–small cell lung | Findings | Yes | No | |||
| Yes | 15 | 11 | 26 | |||
| No | 4 | 116 | 120 | |||
| 19 | 127 | 146 | ||||
| Sensitivity | 78.95% | |||||
| Specificity | 91.34% | |||||
| PPV | 57.69% | |||||
| NPV | 96.67% | |||||
| LR + | 9.114833 | |||||
| LR − | 0.23049 | |||||
| Grant et al72 | No known metastasis Non–small cell lung | No clinical findings | ||||
| Osada et al82 | Non–small cell lung | |||||
| Crane et al71 | New diagnosis Small cell lung | With and without metastasis | Imaging + (CT) | |||
| Findings | Yes | No | ||||
| Yes | 14 | 2 | 16 | |||
| No | 7 | 110 | 117 | |||
| 21 | 112 | 133 | ||||
| Sensitivity | 66.67% | |||||
| Specificity | 98.21% | |||||
| PPV | 87.50% | |||||
| NPV | 94.02% | |||||
| LR + | 37.33333 | |||||
| LR − | 0.339394 | |||||
| Crane et al71 | During follow-up | With and without metastasis | Imaging + (CT) | |||
| Findings | Yes | No | ||||
| Yes | 14 | 4 | 18 | |||
| No | 9 | 48 | 57 | |||
| 23 | 52 | 75 | ||||
| Sensitivity | 60.87% | |||||
| Specificity | 92.31% | |||||
| PPV | 77.78% | |||||
| NPV | 84.21% | |||||
| LR + | 7.913043 | |||||
| LR − | 0.423913 | |||||
| Both | With and without metastasis | Imaging + (CT) | ||||
| Findings | Yes | No | ||||
| Yes | 28 | 6 | 34 | |||
| No | 16 | 158 | 174 | |||
| 44 | 164 | 208 | ||||
| Sensitivity | 63.64% | |||||
| Specificity | 96.34% | |||||
| PPV | 82.35% | |||||
| NPV | 90.80% | |||||
| LR + | 17.39394 | |||||
| LR − | 0.3877445 | |||||
| Hooper et al74 | New diagnosis Bronchogenic | With and without metastasis | Imaging + (CT) | |||
| Findings | Yes | No | ||||
| Yes | 16 | 45 | 61 | |||
| No | 0 | 28 | 28 | |||
| 16 | 73 | 89 | ||||
| Sensitivity | 100.00% | |||||
| Specificity | 38.36% | |||||
| PPV | 26.23% | |||||
| NPV | 100.00% | |||||
| LR + | 1.622222 | |||||
| LR − | 0 | |||||
| Levitan et al79 | New diagnosis Small cell lung | With and without metastasis | Imaging + (CT) | |||
| Findings | Yes | No | ||||
| Yes | 11 | 0 | 11 | |||
| No | 4 | 40 | 44 | |||
| 15 | 40 | 55 | ||||
| Sensitivity | 73.33% | |||||
| Specificity | 100.00% | |||||
| PPV | 100.00% | |||||
| NPV | 90.91% | |||||
| LR + | ∞ | |||||
| LR − | 0.266667 | |||||
| Mintz et al80 | New diagnosis Non-small cell lung | With and without metastasis (Included history) | Imaging + (CT) | |||
| Findings | Yes | No | ||||
| Yes | 3 | 0 | 3 | |||
| No | 5 | 58 | 63 | |||
| 8 | 58 | 66 | ||||
| Sensitivity | 37.50% | |||||
| Specificity | 100.00% | |||||
| PPV | 100.00% | |||||
| NPV | 92.06% | |||||
| LR + | ∞ | |||||
| LR − | 0.625 | |||||
| Tarver et al84 | Initial evaluation | With and without metastasis | Imaging + (CT) | |||
| Findings | Yes | No | ||||
| Yes | 85 | 48 | 133 | |||
| No | 18 | 128 | 146 | |||
| 103 | 176 | 279 | ||||
| Sensitivity | 82.52% | |||||
| Specificity | 72.73% | |||||
| PPV | 63.91% | |||||
| NPV | 87.67% | |||||
| LR + | 3.02589 | |||||
| LR − | 0.240291 | |||||
| Johnson et al77 | Initial evaluation Small cell | With and without metastasis | Imaging + (CT) | |||
| Findings | Yes | No | ||||
| Yes | 10 | 14 | 24 | |||
| No | 2 | 58 | 60 | |||
| 12 | 72 | 84 | ||||
| Sensitivity | 83.33% | |||||
| Specificity | 80.56% | |||||
| PPV | 41.67% | |||||
| NPV | 96.67% | |||||
| LR + | 4.285714 | |||||
| LR − | 0.206897 | |||||
| Jennings et al76 | Initial evaluation Mixed | No clinical findings | ||||
| Butler et al69 | Initial evaluation Lung | No clinical findings | ||||
| Jacobs et al75 | Initial evaluation Lung | No clinical findings | ||||
Abbreviations: LR, likelihood ratio; LR+, likelihood ratio for a positive result (sensitivity/1-specificity); LR−, likelihood ratio for a negative result (1-sensitivity/specificity); PPV, positive predictive value; NPV, negative predictive value
Nine studies included patients only with negative clinical findings, thereby not allowing the calculation of sensitivity and specificity. The negative predictive value (NPV) of a normal clinical evaluation ranged from 0.84 to 1.00. The positive predictive value (PPV) of an abnormal neurological examination ranged from 0.26 to 1.00. Sensitivity and specificity each ranged from 0.38 to 1.00. Toloza and colleagues86 calculated a summary estimate for the NPV of 0.94 (95% confidence interval of 0.91 to 0.96), and some have suggested that brain imaging of carcinoma patients with a normal neurological examination may not be cost effective. For studies that included patients with both positive and negative neurological examinations, their pooled sensitivity and specificity were 0.76 (95% confidence interval of 0.64 to 0.84) and 0.87 (95% confidence interval of 0.74 to 0.94), respectively.86 The pooled positive predictive value was 0.54 (range, 0.21 to 1.00).86 Detection of brain metastases with modern neuroimaging is generally quite effective and accurate. Brain metastases tend to be spherical in shape and often are located at the junction of gray and white matter. Peritumoral edema is generally associated with metastases, and, on imaging studies, the tumors demonstrate peripheral or uniform contrast enhancement. Magnetic resonance or, to a lesser extent, CT imaging is utilized to detect the presence, size, distribution, and quantity of brain metastases in patients (Fig. 6-1). While there is surprisingly little information directly comparing CT and MR scanning, MRI is thought to be superior to CT in detection and radiosurgical planning for the following reasons: (1) MRI permits better detection of smaller lesions; (2) the MRI contrasting agents permit stronger enhancement of metastases; (3) no bony artifact exists in MRI; (4) MRI has fewer partial volume effects; and (5) MRI permits imaging in three different planes.55,87 With MRI, double or triple dose gadolinium-based contrast is more sensitive for detecting brain metastases, but the images must be meticulously reviewed because of the possibility of false-positives with increased contrast doses.88–90 Although the sensitivity of MRI for intracranial brain metastases is superior to that of CT, the specificity for such tumors is not remarkably different between the two imaging modalities.91 Furthermore, there are no studies which demonstrate that MRI is able to identify more patients with brain metastases compared with CT scanning, nor is it clear that detection of more and smaller lesions by MRI translates into a clinically meaningful difference in terms of survival.92 Recently, the use of positron emission tomography (PET) in radiosurgical planning has been advocated.93,94 In theory, such information may be useful for stereotactic planning and dose selection as well as postradiosurgical follow-up to assess tumor response.93–97 Also, on postradiosurgical imaging, PET and magnetic resonance spectroscopy (MRS) scanning may be useful in distinguishing between radiation effects and tumor recurrence.95–98 In one report, the sensitivity and specificity of 18 F-fluorodeoxyglucose (FDG) PET in distinguishing recurrent brain metastasis from radionecrosis were 65 and 80%, respectively.95 However, with MRI coregistration, FDG PET had a sensitivity of 86% while still maintaining a specificity of 80%.95 In a similar type of study, the sensitivity, specificity, and accuracy were 75, 93.9, and 91.2% for FDG PET and 100, 65.3, and 70.2% for MRI, respectively.99 Coupling the sensitive but nonspecific MRI with the specific but insensitive FDG PET will be most useful in terms of diagnostic accuracy to help differentiate radionecrosis from tumor progression. However, our own experience with the utility of PET scanning has been less than sanguine.100 Because of the limited number of high-quality studies, the clinical efficacy of PET scanning, MR spectroscopy, and other imaging modalities (e.g., thallium SPECT) in stereotactic radiosurgery remains the subject of investigation.101,102
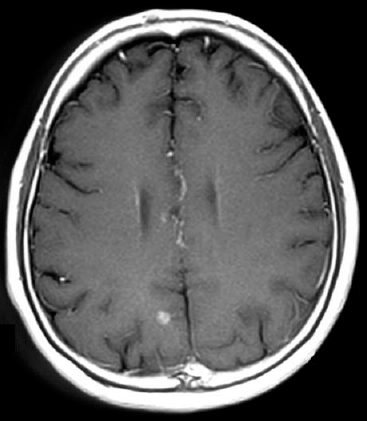
Figure 6-1 A computed tomography scan demonstrating a brain metastases after administration of intravenous contrast.







