and Róbert Bódizs2
(1)
Institute of Experimental Medicine and Institute of Neuroscience, Budapest, Hungary
(2)
Semmelweis University Institute of Behavioral Science, Budapest, Hungary
Abstract
In this chapter, we will deal with data accumulated about the arousal-driven spontaneous and evoked phasic events. We are looking for characteristics of spontaneous and elicited phasic changes, regularities in their occurrence, and the impact of their existence on the course of sleep. Summarizing the results of earlier works about PAT, K-complexes, and CAP phenomena, a new approach has been used in their systematization. First, we have differentiated arousal-like and sleep-like spontaneous and elicited responses and investigated the relation of these two types with dynamic changes in the course of night sleep. We differentiated these phasic changes from tonic states of sleep and analyzed the interrelationships of the phasic and tonic changes.
Keywords
Phases d’activation transitoireK-complexesMicro-arousalsReactive delta wavesCyclic alternating patternDijk and Kronauer wrote in a comment on “Models of sleep regulation: successes and continuing challenges” in 1999 that “although sleep models may describe adequately global sleep patterns and their circadian modulation, detailed modelling of the frequent short awakenings from, and the subsequent transitions back to sleep, as well as the variation of the propensity to awaken across the ultradian non-REM-REM cycle, is not addressed. Incorporation of these aspects of sleep in mathematical models of sleep regulation may improve further our understanding of key aspect of sleep regulation, that is, its timing” (Dijk and Kronauer 1999).
3.1 “Phases d’Activation Transitoire” (PAT) (First Recognition that Arousal-Like Events Are Standard Constituents of NREM Sleep)
The phenomenon of spontaneous recurrent arousals with characteristic EEG changes and polygraphic signs, without behavioral awakening was first described Schieber et al. (1971) named at that time as “phases d’activation transitoire” (PAT). The criteria for PAT in NREM sleep given by Schieber et al. (1971) were the following: “Increase in EEG frequencies in conjunction with decrease of amplitudes, disappearance of delta waves and spindles, transitory enhancement of muscle tone or phasic appearance of groups of muscle potentials, movements of the limbs or changes in body posture, transitory rise in heart rate.”
The duration of these changes varied from some seconds to more than 10 s. Temporary activation is followed by deactivation leading to a biphasic character of the phenomenon (Fig. 3.1). The term was modified by several workers in the last years and used in the context of physiologic and pathologic studies, with more or less the same meaning and criteria (Quattrochi et al. 2000; Sforza et al. 2002).
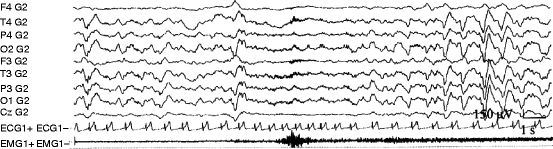

Fig. 3.1
Example of a spontaneous PAT phenomenon during NREM sleep. Sleep EEG (stage 3) became suddenly desynchronized with appearance of faster rhythms concomitant with transitory increase in muscle activity and heart rate. After the phasic event, a rebound-like increase in EEG slow wave activity can be seen parallel with transitory heart rate decrease
The occurrence of PAT negatively correlates with the depth of sleep, occurring more frequently in superficial than in deep sleep with the highest incidence during REM sleep and stage 1 and appearing less frequently during stage 3 and 4 (Fig. 3.2). Erhart and Muzet (1974) in the same research group have studied the elicitability of PAT by acoustic and thermal stimuli. They had the fundamental finding on the existence of the same arousal pattern (evoked PATs) in response to artificial stimuli as they appear spontaneously. They have registered beside the evoked PATs spontaneous PATs as well but in diminished number compared to the nights without stimulation. Therefore, they assumed a control mechanism regulating the number of arousals during the night sleep.
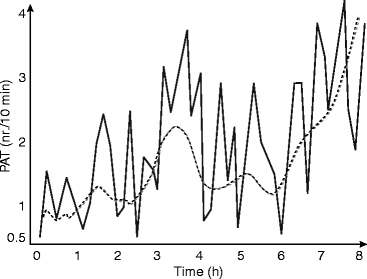

Fig. 3.2
The evolution of PAT (phases d’activation transitoire) events during a night sleep in ten young healthy volunteers (After Schieber et al. 1971)
PAT is the first systematically studied phasic event identified with arousal/activation recurring during sleep. It has been suggested by the Brussels scholars, that spontaneous activation periods are related to endogenous impulses of the reticular activating system.
3.2 K-Complexes: The First Hit in Recognition of Reactive Deltas in NREM Sleep
The first observation proving the elicitability of delta waves was the discovery of K-complexes (Blake and Gerard 1937; Loomis et al. 1939; Davis et al. 1939). Researchers were already fascinated by the resemblance of the spontaneously occurring isolated slow waves to those ones they were able to induce by external stimuli delivered during sleep. Two recent reviews are available to have a broader overview beyond the scope of this book (Colrain 2005; Halász 2005).
K-complexes during sleep appear at 5 months of age. K-complexes are best distinguishable in stage 2 sleep, as a spontaneously occurring phasic events, but it is possible to elicit them by sensory stimuli as well. In deeper stages, high-voltage slow waves practically absorb K-complexes but with averaging, the same kinds of K-complex-like waves have been elicited in stage 3–4 sleep (Ujszászi and Halász 1988; Bastien and Campbell 1992; Niiyama et al. 1995). The frequency of spontaneous K-complexes is between 1 and 2/min in most studies (Halász et al. 1985).
The components of the K-complex are in close relationship to or can be identified with late components of sensory evoked potentials in sleep, as shown in studies on K-complexes evoked by acoustic stimuli (Ujszászi and Halász 1988; Bastien and Campbell 1992; Niiyama et al. 1995; Riedner et al. 2011). Components of the potential complex that occur in response to short tone pips in NREM sleep stages 2–3 are generally accepted as follows: N100, P200 (or 250), N300 (or 350), P400, N550, P900, N1500, and P1900 (Fig. 3.3). It was proved that N350 could be present even when N550 and P900 do not follow or are absent (Bastien and Campbell 1992) and held to be identical with the “vertex sharp wave.”
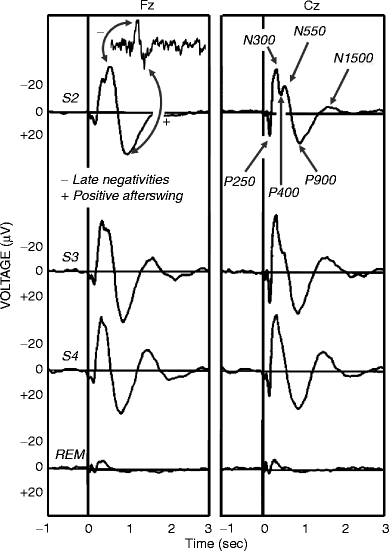

Fig. 3.3
K-complexes as auditory evoked potentials in different stages of human sleep reflected in Fz and Cz electrodes. A spontaneous singular event sharing the main features of the evoked and averaged events (late negative components and positive afterswing) is shown in the top left of the figure. Components of the auditory evoked potentials are defined on the top right. Note the persistence of the main features and the similarity between spontaneous and evoked K-complexes. S2 stage 2, S3 stage 3, S4 stage 4, REM rapid eye movement sleep
The first studies of K-complexes showed that they are elicitable by all modalities of sensory stimuli (Bastien and Campbell 1992; Niiyama et al. 1995; Roth et al. 1956; Sallinen et al. 1994), but most easily by acoustic stimuli and are accompanied by autonomic discharges identical to those seen for arousals (Ackner and Pampiglione 1957; Fruhstorfer 1971, 1995; Hornyak et al. 1991; Johnson and Karpan 1968; Sassin and Johnson 1968; Sforza et al. 2000, 2002; Takigawa et al. 1980).
The distribution and amplitudes of the late components of the K-complex proper (N350-N550-P900) vary according to three factors: topography, level of sleep, and information content (novelty and meaning) of eliciting stimuli (Ujszászi and Halász 1988). The whole complex is not homogeneous but probably represents a cascade of events originating from sources of different topography that are mobilized by different processes. They are activated in a certain order provoked by the nature and context of the eliciting stimuli (Halász 1993).
Continuously or periodically applied random acoustic stimulation increased the number of K-complexes and simultaneously decreased the number of the spontaneous K-complexes (Halász 1982; Halász et al. 1985). (This issue will be elaborated in Chap. 4.)
While several data clearly show that K-complexes have arousal response properties, other studies indicate that K-complexes are “fore runners” of NREM delta activity, their amplitude increase after sleep deprivation, similarly to deltas (De Gennaro et al. 2000; Nicholas et al. 2002; Peszka and Harsh 2002), and they are held to be sleep-promoting or preserving agents (Wauquier et al. 1995). The distribution of K-complexes from cycle to cycle across the night proved to show parallel course with homeostatic decay of slow waves (Halász et al. 1985; Rajna et al. 1983).
We have emphasized repeatedly that K-complexes have “Janus-faced” features based on their arousal dynamic contrasted with their sleep-maintaining, anti-arousal properties (Halász et al. 1985; Halász 2005).
Bastuji et al. (1995) developed the forced awakening method. In this paradigm, subjects were questioned about quantitative and qualitative aspects of stimulus recall evoked by oddball-type stimuli in parallel with recording of the evoked cortical responses, after being aroused by the stimuli from naps. In subjects whose quality of recall was excellent, P300 waves were indistinguishable from those obtained before sleep. When P300 was found attenuated, delayed, and desynchronized, recall was quantitatively degraded and P300 was concomitant to or replaced by sleep negativities (varieties of late negative components being part of the K-complex) in subjects in whom stimulus recall was severely degraded or absent. They concluded that K-complex-analogue sleep negativities have two aspects being, on the one hand, arousal driven and, on the other, erasers preventing accurate memory encoding and retrieval of the stimulus, consequently promoting sleep. Thus, the same Janus-faced nature of K-complexes was stressed by them similarly to our previous work.
Earlier studies showed already that K-complexes and delta waves have prefrontal-frontal localization emanating from a wide bilateral field (Ujszászi and Halász 1988; Bastuji et al. 1995; Colrain et al. 2000; Bastien et al. 2002). The scalp topography of K-complexes evoked by acoustic and respiratory stimuli was similar (Gora et al. 2001). Also, the large positive afterswing like P900 component reached an amplitude-maximum over the fronto-central areas (Ujszászi and Halász 1988; Bastien and Campbell 1994; Cote et al. 1999) and the frontal dominance became more prominent with deepening of sleep to stages 3–4 NREM. The frontal maximum of delta waves during deep sleep (stages 3–4) was evident long ago and is still supported by several power spectra studies of NREM sleep (Horne 1993; Cajochen et al. 1999; Finelli et al. 2001; Marzano et al. 2010).
Czisch and coworkers (2009) using EEG-fMRI have found maximal activation during sound tone evoked K-complexes in the middle frontal gyri and cingulate areas. In another recent study, Reidner et al. (2011) applied a more sophisticated EEG source analysis with sLORETA method and confirmed that the bilateral anterior cingulate, middle frontal, inferior frontal orbital, and rectal gyri were the most pronounced areas in terms of relative current during K-complexes evoked by different (auditory, somatosensory, and visual) stimulation. Furthermore, they were able to detect a modality-specific activation (by relatively increased current) in specific cortical areas in accordance with the modality of the applied type of stimulation at the 550 ms slow negative peak of the evoked K-complex.
These results provide a direct link between modality-specific sensory information processing and diffuse nonspecific K-complex-like slow wave responses during NREM sleep.
Recently, Jahnke et al. (2012) found in another fMRI analytic study found a wide spontaneous K-complex-associated network reflected by BOLD positive signal changes in subcortical (brain stem, thalamus), cerebellar, sensory (auditory and visual), motor midline (anterior and midcingulate gyrus, precuneus), and other regions which form part of the default mode network. They emphasized that the primary auditory cortex was the first cortical region to be influenced during the K-complex. Their interpretation is very much the same as in our earlier works:
K-complex embodies an arousal with subsequent sleep guarding counteraction that might on one hand serve monitoring of the environment with basic information processing and on the other hand protect continuity of sleep and thus its restoring effect.
In another recent paper, Koshaka et al. (2012) reported on transient activation of the ventral brain stem preceding the K-complex by detection of auditory brain stem-evoked responses and a sustained activation of the dorsal brain stem outlasting the K-complex. Thus, it is suggested that K-complexes are triggered by the activation of the brain stem.
The question how primary sensory pathways are able to activate in NREM sleep the nonspecific slow wave response is presently unknown. One possibility would be the parallel activation of the thalamocortical system producing slow waves when arousal system is shut down. Another assumption can be the secondary involvement of larger cortical fields via cortico-cortical connection after the initial cortical modality-specific activation. A further approach is outlined in Sect. 6.2.
Intracranial distribution of K-complexes obtained by stereo-electroencephalographic recordings has been detected recently. Congruently with the scalp topography and source analytic studies the intracranial distribution of K-complexes has been found to be maximal over the anterior medial and superior frontal cortices (Wennberg 2010).
A very important new line in the K-complex research has been started by the report of Amzica and Steriade (1997) showing the relation of K-complexes and slow oscillation of NREM sleep below 1 Hz and later by Cash et al. (2009) pointing out a particular relationship with the large negative main component of K-complex and the down state of the slow oscillation (Fig 3.4).
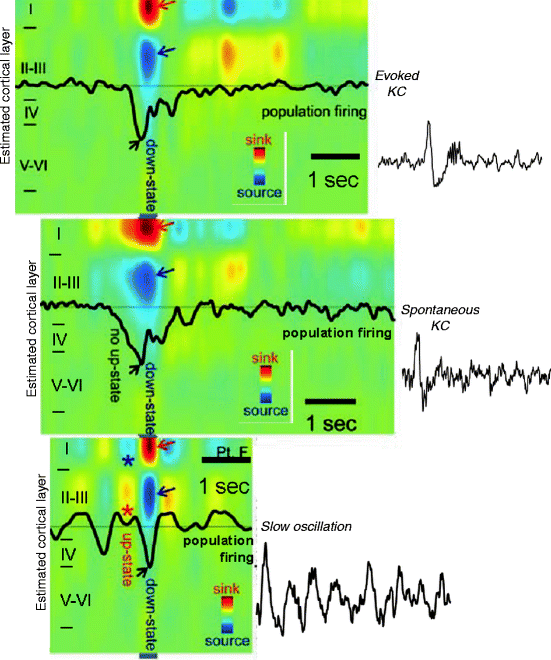

Fig. 3.4
The cortical down state as a common denominator of K-complexes and slow waves. Evoked and spontaneous K-complexes of stage 2 sleep are common in being based on singular down states, while the down state of the slow oscillation of stages 3–4 is embedded in alternating up-down state sequences. The figure is the result of coregistered multiunit activity and current source density with an intracortical multimicroelectrode in human subjects. Red arrow indicates inward currents (sink) in superficial cortical layer and blue arrows show outward currents (source) in layers II–III. Black arrow indicates decreased neuronal firing (Modified from Cash et al. 2009)
In stage 2 sleep, 18–20 % of K-complexes are followed by long lasting changes in the ongoing EEG. They are accompanied by other rhythms such as K-delta, K-alpha, and K-spindle according to the nature of the associated rhythm (Raynal et al. 1974; Halász and Ujszászi 1991; MacFarlane et al. 1996).
Although isolated K-complexes are present in stage 2 mainly at the more superficial level, the majority of them are part of a longer phasic event called by us as “micro-arousals.” The term micro-arousal for these changes was used to designate those phasic EEG events which were not associated with awakenings regardless of their desynchronizational (fast, low amplitude) or synchronizational (sleep response-like) morphology and regardless of their connection with autonomic phenomena or some sort of behavioral arousal (Halász et al. 1979).
3.3 Nature of Phasic Events and Two Basic Type of Reaction to Phasic Input During NREM Sleep
The term “phasic event” was first coined for different transient REM sleep EEG phenomena (like ponto-geniculo-occipital spikes or rapid eye movement bursts), which differ either in amplitude or pattern, or most frequently by both of them from the ongoing background activity (Moruzzi 1963). Later this term became generally used for other transient event (like PAT, vertex waves, K-complexes, slow wave groups) observed during different stages of NREM sleep. The recognition of the universal recurring presence of phasic events in NREM sleep revealed that these events, although they might appear seemingly without any eliciting stimulus, are elicitable by mild arousing stimuli which did not awake the sleeper. Although the potential to evoke a phasic event proved to be different according to the different modalities of the stimuli, the most essential observation was that elicitability was not modality specific. Another essential feature of them is the association with more or less autonomic activation (measured usually by heart rate elevation and motor response/increase of EMG activity). This, on one hand spontaneous, on other hand reactive feature of phasic events, raised the assumption that they might be evoked by unnoticed internal (within the body or brain), or external eliciting stimuli.
Whatever the input was, the reactive events belonged to two types (Fig. 3.5). One group of them behaved according to the classical expectation of an arousal response: increase of frequency and decrease of amplitude. It is the PAT prototype. The other group of phasic events was characterized by mor sleep than arousal like response with K-complexes, and slow groups, without or with lower intensity autonomic and motor response, the EEG answer was characterized by a more sleep than arousal like response with K-complexes, slow wave groups. Therefore, the “arousal-like” nature of this type was from the beginning questioned raising debates among sleep researchers about the nature of this Janus-faced reactivity being partially linked to arousal and partially to sleep induction or maintenance (Wauquier et al. 1995; Hirshkowitz 2002).
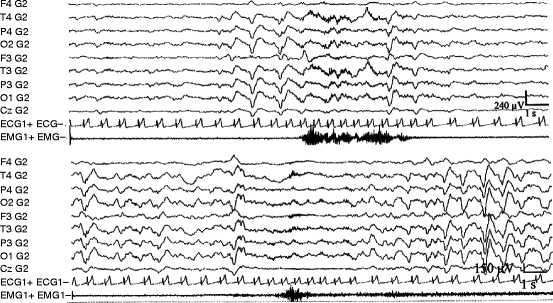

Fig. 3.5




Characteristic examples of synchronizational (a) and desynchronizational (b) phasic changes during NREM sleep. The essential contrasting features are seen in the EEG parts: in (a) a sudden transitory increase of slow wave activity, while in (b) the transitory appearance of fast rhythms suppressing the ongoing slow wave activity can be observed. Simultaneous autonomic and motor activation are almost the same during both responses
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








