Fig. 1.1
Mesocorticolimbic system. Left axial high field MRI slice overlaid with anatomical structures of the executive-behavioral system, containing predominantly, dopamine (blue) or acetylcholine (orange) neurons. Right mesocorticolimbic system
1.2 Anatomo-Functional Correlates of the Executive-Behavioral System
The frontal, the temporal and the limbic lobes, coupled with basal ganglia , thalamus, hypothalamus, and upper midbrain nuclei are the main structures modulating behavioral phenotypes, and are all or part linked with psychiatric disorders . The cerebellum could be involved in psychiatric symptoms, through the cerebello-thalamo-cortico-pontine loop , in the posterior a fossa, and the cerebellar cognitive affective syndromes and others cognitive and affective disorders [15, 69].
The core system supporting neural correlates of psychiatric disorders is the executive-behavioral system involving the prefrontal and the cingulate cortices, and the rest of the limbic system, which includes the limbic lobe (Fig. 1.2). From the clinical experience, the whole frontal lobe could be involved in the executive-behavioral system, however this has to be interpreted cautiously [4], even though recent data shows that the supplementary motor area of medial motor cortex could participate to action-monitoring system, adjusting behavior according to the result of action [8]. Functional imaging should help to segregate functionalities supporting executive-behavior functions within the frontal lobe [102].
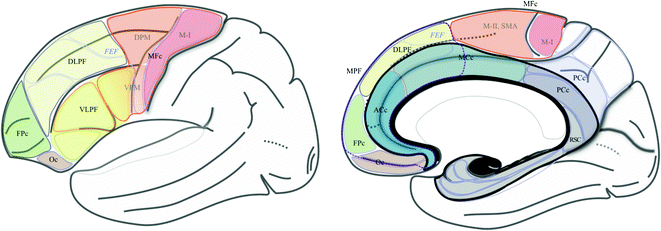

Fig. 1.2
Frontal and cingulate functional territories, and the limbic lobe. Segregation of frontal and cingulate cortices into functional territories [30, 82, 107], semi-schematic drawing; the frontal cortex is subdivided into motor and prefrontal regions. The motor cortex is made up of the primary motor (M-I; B4), dorsal premotor (DPM; B6), ventral premotor (VPM; B6, 44 and 45) and medial premotor (or supplementary motor area; MII-SMA; B6) cortices. The prefrontal cortex is made up of the ventrolateral prefrontal, the dorsolateral prefrontal (DLPF), the frontopolar (FPc), the orbitofrontal and the medial prefrontal cortex, which involves, (1) the medial part of the DLPF and the FPc cortex, and (2) the anterior cingulate cortex (ACc) and the rostral part of midcingulate cortex. The cingulate cortex is made up of ACc, MCc, posterior cingulate and retrosplenial cingulate cortices. The limbic lobe [19] is made up of the limbic (B27, 51 and 34) and paralimbic cortices (grey), the subcallosal and cingulate gyri, the isthmus and the parahippocampal gyrus, and the intralimbic gyrus (black), with 3 segments, anterior, superior and inferior or hypocampus (fine white line)
The executive-behavioral system supports the so-called “emotional brain” and “social brain” [24, 52], coming from pioneering works of Broca introducing the term “grand lobe limbique” [11], Papez [79] proposing the concept of corticothalamic correlate of emotions, and MacLean [58] extending Papez’s work with the visceral brain (Fig. 1.3). A lot of connections between these structures are known, although the functionality, physiologic and pathophysiologic, of circuits is not fully mastered.
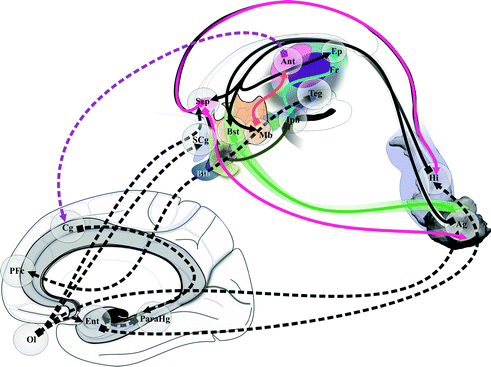

Fig. 1.3
Broca’s, Papez’s and Maclean’s contributions to the emotional, social and limbic brain
Broadly elements of executive-behavioral system are pushed aside by WM fascicles of the internal capsule, separating two groups: (i) the medial group, hypothalamus, subthalamus, thalamus, head of caudate nucleus , accumbens nucleus; (ii) the lateral group, hippocampus-amgydala complex , lateral striatum (putamen and tale of caudate nucleus ), pallidal complex, substantia innominata , claustrum and insula. GM territories lined by arched fascicles connect the medial and lateral groups: superior (dorsal) system, cingulum with the cingulate, hippocampal, para hippocampal (including the entorhinal area), subcallosal and anterior intralimbic gyrii, olfactive fascicle, stria terminalis, fornix, and body of caudate; inferior (ventral) system, nucleus of ansa lenticularis and ansa lenticularis, extended amygdala, diagonal band of Broca and ventral pallidum. Commissural structures linked right and left elements of the executive-behavioral system, such as the corpus callosum, the fornix and the anterior commissure. The Fig. 1.4 summarizes anatomic elements of the executive-behavioral system.
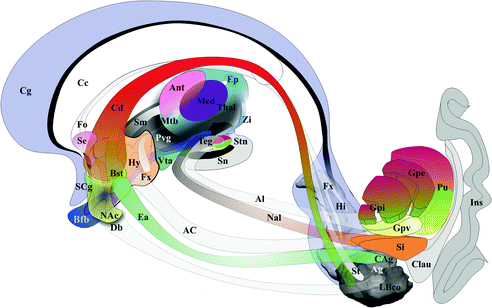

Fig. 1.4
Anatomic elements of the executive-behavioral system
The cingulum (Fig. 1.5) is made up of three fascicles [89]: anterior connecting the anterior perforate substantia with the frontal lobe, the horizontal connecting frontal, limbic and parietal gyrii; the posterior connects medial and lateral occipito-temporal gyrii with the temporal gyrus. Recent advances have added new insights into the cingulum bundle, showing the multiple connection fibers merged [46]. In monkeys the cingulum also contains fibers connecting the prefrontal dorsolateral cortex and the hippocampal formation [31]. The distal, rostral, anterior cingulate is a part of the sub cingulo-callosal region that includes also the subcallosal (or subcallosal cingulate) gyrus and the carrefour olfactif of Broca (paraolfactory area). The gyrus rectus merges with the anterior cingulate and the subcallosal gyrus within the carrefour olfactif [89]. The organization of the WM of the sub cingulo-callosal region illustrates the complexity of connections, allowing the prefrontal cortex to connect with the lateral and the medial groups of elements of the executive-behavioral system (Fig. 1.6). The fornix connects the hypothalamus (mammillary and tubero mammillaris), the septum, and the habenula, with the Amon horn (alveus; medial) and the gyrus dentatus (fimbria; lateral). The olfactive fascicle connects the posterior septum and cingulate through the corpus callosum, with the anterior septal nuclei and the anterior perforate area, and continues to the amygdala, the substantia innominata and the uncus, through the diagonal band of Broca [89]. The stria terminalis connects the septal and paraseptal region (or bed of the stria terminalis) with the amygdala; the extended amygdala, a network of sparse cells and connections within the rostral mediobasal forebrain, located laterally to the hypothalamus and below the lenticular nucleus, bridges the bed nucleus of stria terminalis and the centromedial amygdala [27, 26]. The hypothalamus, made of 11 nuclei, had a large connectivity with the cortex and the deep brain [55] (Fig. 1.7), some features seems specific: dorsomedial nucleus mostly connected with the medial thalamus and the midline gray substance; ventromedial area (ventromedial nucleus and the adjacent tuberomamillaris nucleus) strongly connected with the prefrontal cortex; preoptic region predominantly connected with the septal region, the substantia innominata of Reichert and the anterior perforate region. The posterior part of hypothalamus belongs to the ventral tegmental area (see [23]), which is closely related functionally with the substantia nigra compacta and the retrorubral nucleus (or retrorubral field), so called VTA-nigral complex in rodents [22]. The VTA is subdivided into centromedial and lateral parts, which project respectively to the core and shell of accumbens nucleus; VTA also projects to the dorsal striatum, the septum, the lateral habenula and the amygdala, and has reciprocal connections with cortices in particular the pre frontal [22, 76, 104]. The thalamus is made of numerous nuclei, which can be labelled in a simplified way according to the human brain orientation and segregated into 9 groups: anterior or oral, dorsal, intermediate, ventral, medial, laminar, posterior or caudal, superficial and related nuclei [54] (Fig. 1.8). The functional limbic territory of the striato-pallidal system is rostro-ventral and is called the ventral pallidum, internal and external, and the ventral striatum [34, 47]. The internal ventral pallidum is well-defined below the anterior commissure, although laterally it overlaps with the globus pallidum external and internal, and the substantia innominata . The accumbens nucleus, also called ventral striatum [43], can be separated into two functional territories, the core, dorsal and the shell, ventral [108] (Fig. 1.9). The ventral striatum also includes the olfactory region of the anterior perforate substantia [104]. The claustrum origins from the cortex [85, 89] and is connected ventrally with fibers of the anterior commissure and the olfactive fascicle [89]. The insula is, inter alia, limbic [68]. The striato-limbic substantia innominata of Reichert has numerous connections (Fig. 1.10) and contains the nucleus basal of Meynert (medial) merging with the nucleus ansa lenticularis [39, 72, 81, 89, 94]. The thalamo-tegmental reticular system makes the link between sensori-motor inputs and executive-behavioral system. It is made up notably of the centromedian-parafascicular complex and the reticular formation of the brain stem, and has numerous connections with the cortico-striato-pallido-subthalamo-thalamo-cortical loop (see e.g. [93]). The effectiveness of vagus nerve stimulation alleviating symptoms of drug-refractory depression could be explain by the role of thalamo-tegmental reticular system [71]. The gabaergic reticular nucleus of the thalamus, placed between cortices and thalamic nuclei, could participate to the pathophysiology of schizophrenia [21].
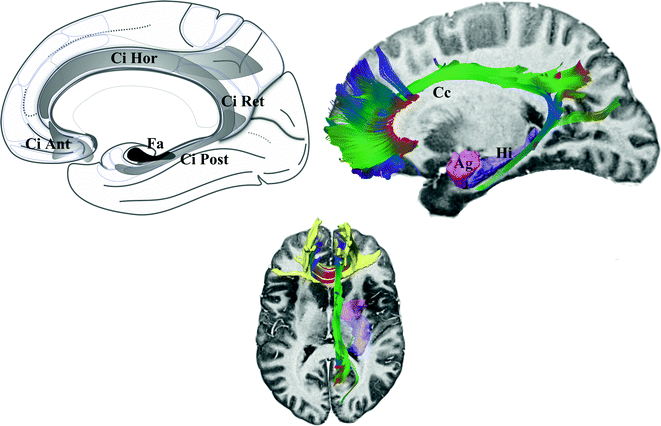
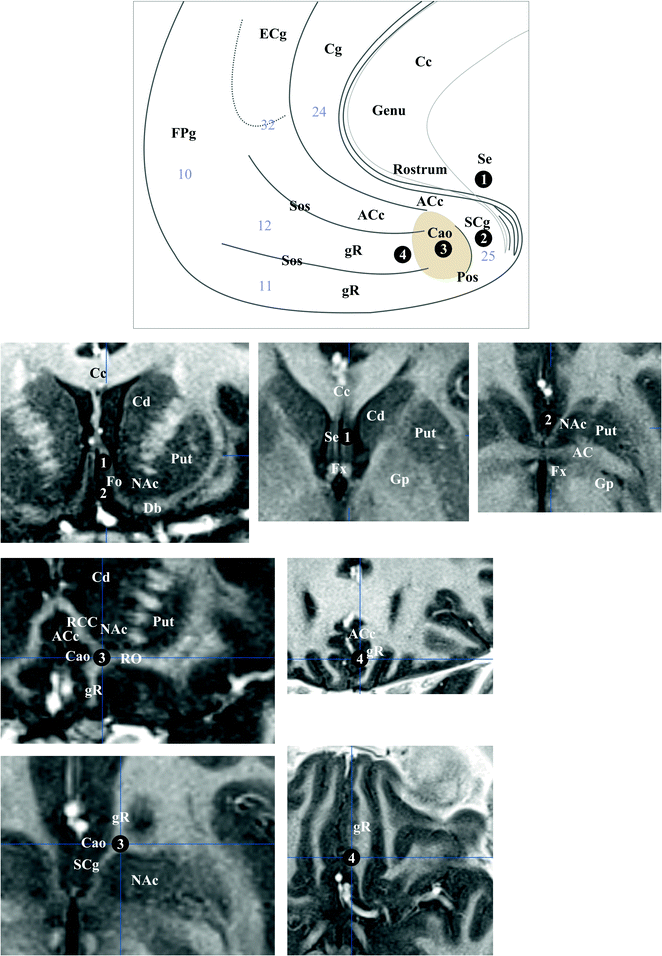
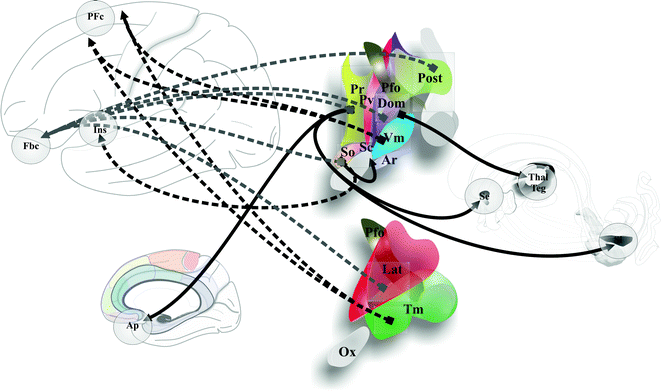
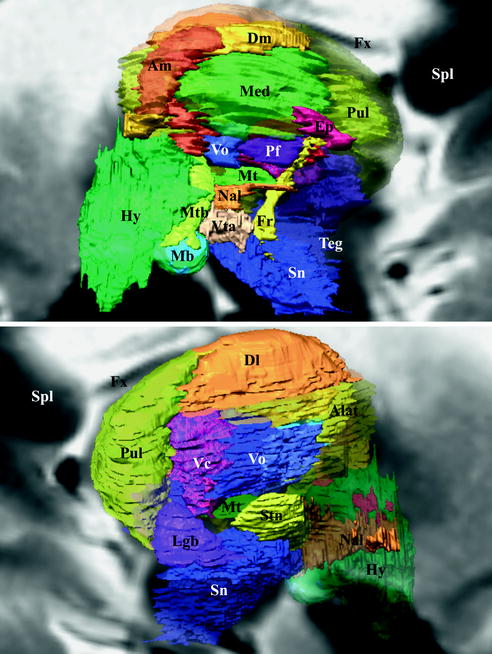
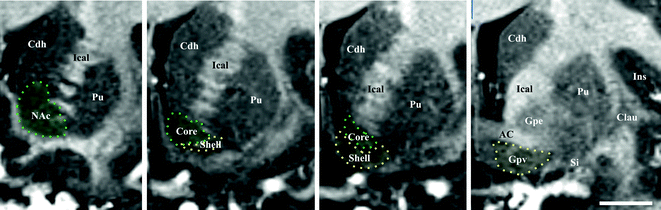
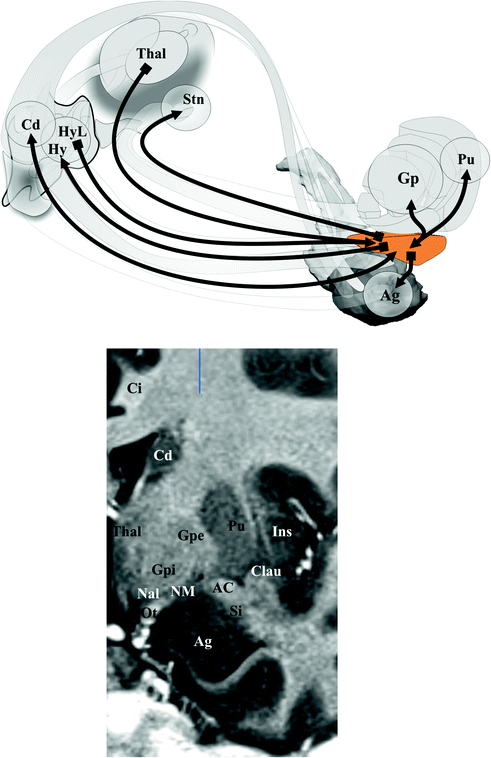

Fig. 1.5
Cingulum. Left fascicles of the cingulum. Right DTI tractography of the cingulum showing different components; note that anterior commissural fibers of the beak of callosum (yellow) merge with anterior fibers of the cingulum

Fig. 1.6
Sub cingulo-callosal region. Top semi-schematic drawing, medial view, bottom MRI slices going through 1 and 2 (top row; left, coronal, intermediate and right axial), 3 (left column; top, coronal, and, bottom, axial) and 4 (right column; top, coronal, and, bottom, axial). Brodmann’s areas are specified (light gray numbers)

Fig. 1.7
Hypothalamus connections. Cortical and deep brain connections

Fig. 1.8
Thalamus, hypothalamus and subthalamus. Medial (top) and lateral (bottom) views of reconstructed anatomical structures from high-field MRI of human brain (background, sagittal MRI slices)

Fig. 1.9
Accumbens nucleus. Coronal MRI sections of the nucleus accumbens (from left to right, from rostral to caudal). Delineation of core and shell (dotted lines) is done according to [108]; note that the caudal shell is mixed up with the ventral pallidum. White bar = 10 mm

Fig. 1.10
Substantia innominata. Top connections; bottom MRI topography (coronal slice)
1.3 Anatomo-Functional Correlates of Psychiatric Disorders
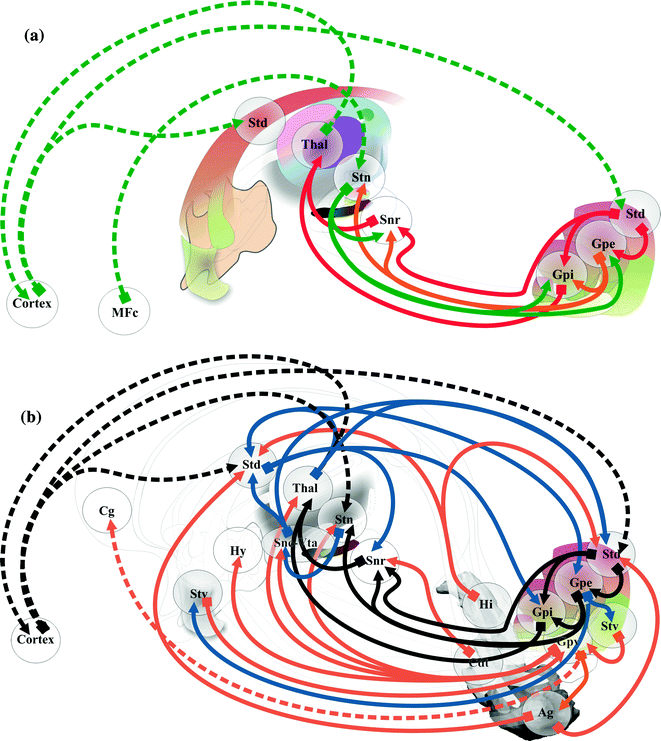
The concept of emotional brain had progressively involved all deep brain structures, in particular the sensorimotor’s and notably their limbic part. The importance of interactions between the cortex and deep GM nuclei in psychiatric disorders is highlighted through the so-called cortico-basal circuitry. Compulsive and repetitive behavior observed in obsessive compulsive disorder (OCD) and autism spectrum disorders, are the most emblematic symptoms linked with the cortico-striato-pallido-subthalamo-thalamo-cortical loop [101]. The dorso-ventral segregation of basal ganglia circuits into limbic-emotional, ventral, associative-cognitive, intermediate, and sensory-motor, dorsal, systems exemplifies the interrelations of basal ganglia and thalamus with the frontal cortex during behavior control, notably emotional [34, 35, 38]. For instance, the sensation of pleasure, emotional, would be mediated by a ventral striato-pallidal circuit, while motivation (‘‘wanting’’) used a dorsal striato-GPi/SNr circuit [62]. The sensory-motor circuitry, especially the hyper direct pathway between cortex and STN [73] (Fig. 1.11a), plays a role within the cortico-sub-cortical circuitry involving a lot of ventral connections (Fig. 1.11b). Clinical reports of chronic electric stimulation (DBS) illustrate the involvement of basal ganglia into the executive-behavioral system, and in particular the STN concentrating the three functional territories within a very limited volume. DBS of the anterior and medial part improves compulsive behavior in OCD patients [60], and DBS of the sensori-motor part can provoke symptoms of depression in severe Parkinson’s disease [99]. In severe Parkinson’s disease, stimulo-induced hypomania was reported with stimulation of the limbic part of STN [59], but also when contacts are within the substantia nigra [103]. Pallidal DBS can also provokes hypomania in parkinsonians [70].
Known and hypothetical neural correlates of emotional states of mood, such as depression and anxiety (negative valence) and mania and hypomania (positive valence), serve as models of circuitry of mood-affective disorders, e.g. in depression, OCD and bipolar disorders. Depressive mood disorders are associated with chronic stress, supported by the hypothalamic–pituitary–adrenal axis (paraventricular nucleus) [57, 96]. Chronic stress is also linked with obesity, thus metabolism control and mood interacts [42] notably through the leptin pathway in the hypothalamus (paraventricular and arcuate nuclei) and the hippocampus [29]. Hypothalamus is a key actor of regulation of food intake (see neuromodulation examples [67, 110]) controlling metabolism and behavior. Effects of STN and internal pallidum DBS also show the involvement of basal ganglia in food intake; weight gain is explained, inter alia, by modifications of metabolism and eating behavior [88]. Anxiety and fear fall when pathologic in anxiety disorders, such as the generalized anxiety disorder. Substance disorders, abuse and dependence refer to persistent, compulsive and repetitive behaviors, and associated harm, thus consequently to rewarding. Mood and reward are not independent as inferred from food intake behavior [109].
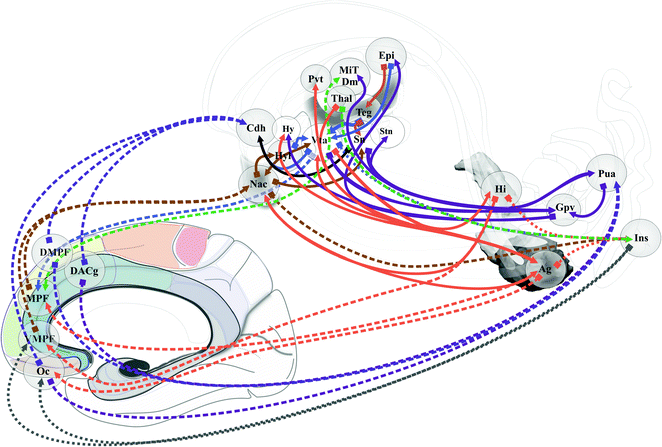
Emotion perception could be supported by two systems [83]: ventral, amygdala, insula, ventral striatum and ventral anterior cingulate and prefrontal cortices; dorsal, hippocampus, dorsal anterior cingulate and prefrontal cortices. Processes of emotion regulation could support models of bipolar disorder [84] (Fig. 1.12). Prefrontal cortex participates differently according to the type of regulation: automatic emotion regulation by medial prefrontal along with hippocampus and para hippocampus cortices; voluntary emotion regulation by lateral prefrontal cortex [90]. The sub cingulo-callosal prefrontal region, modulating mood, is atrophied at the expense of glia in major depressive disorder and bipolar disorder, and the metabolic activity increased in major depressive disorders diminishes after treatments [17, 18, 64]. DBS within the sub cingulo-callosal region modulates mood and anxiety, but also anorexia nervosa-related OCD [56]. More globally the emotional-limbic brain, including medial thalamic and sub-ventricular relays, modulates mood and related disorders [87], involving most basal ganglia circuitry [62], up to the insula [5]. In the core of the executive-behavioral system, is the hippocampus-amgydala complex . The hippocampus mediates temporo-spatial encoding and recalling of events, and is co-activated with the amygdala during fear conditions; short connections link functionally amygdala and hippocampus [27]. The amygdala participates to a variety of emotion processes, during fear, reward, attention, perception and explicit memory; it is connected to hippocampus, cortex, thalamus, hypothalamus, ventral striatum, periaqueductal gray and neurovegetative systems [53]. It has been proposed that the bed nucleus of the stria terminalis participates to alcohol abuse disorder [48, 97]. Finally the amygdala should play a role in psychosis, in particular though dopamine inputs [25]; most basal forebrain structures could be involved. Reward is a positive emotional stimuli, such as food, sex and social interaction [91], reinforcing behavior, leading to conditioned behavior [45]. The reward circuit encompasses a lot of mediobasal structures [45, 91], such as VTA, hypothalamus, ventral striatum and medial prefrontal cortex, modulated in particular by dopamine release in the ventral striatum, the amygdala, and the prefrontal cortex [10, 25, 97] (Fig. 1.13). The insula is also involved in addiction [28, 95]. DBS of the anterior limb of the internal capsule, in the vicinity of ventral striatum and pallidum, accumbens nucleus and lateral hypothalamus improves OCD patients and treatment-resistant depression, on both compulsive activity and depression [33, 61]. Right hemisphere dominance of mood and reward controls could exist [55, 92]. Stimulo-induced, fear, panic and smile were observed when contact are positioned in the ventral part of the anterior limb—accumbens nucleus region [77]. DBS of the accumbens nucleus in OCD alleviates symptoms of depression, anxiety and anhedonia [7].
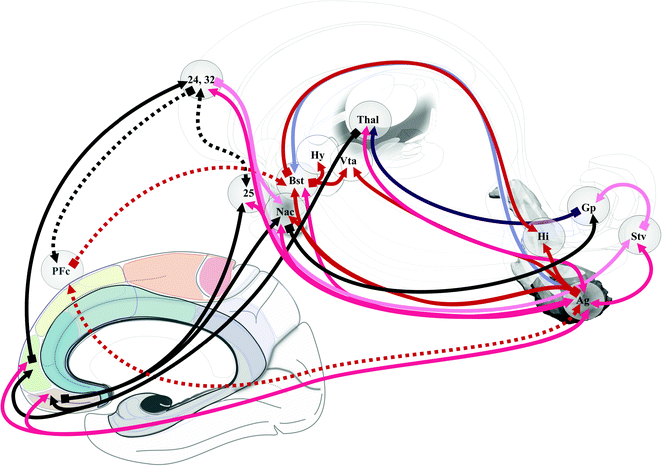

Fig. 1.12
Neural correlates of bipolar disorder. According to Strakowsky et al. [98]. Numbers specify Brodmann’s areas
Neural correlates of memory (Fig. 1.14) were first described at the beginning of the 20th century when memory defects were analyzed in clinics (see [6] for a review); they involved the mammillary bodies, the fornix and the hippocampus, often called Papez’s circuit . Recent clinical studies have shown memory enhancement when stimulating entorhinal cortex [100] and fornical area [51]. The nucleus ventral tegmental of Gudden would participate to memory processing [106]; the anterior thalamus could be an integrative relay from hypothalamus and hippocampus [1]. The memory circuit is at the core of the executive-behavioral system where two structures are particularly involved in memory process: the medial prefrontal cortex [20] and the amygdala for emotional dimension [66].
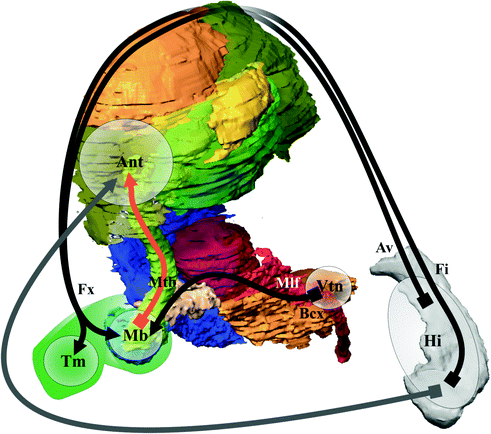

Fig. 1.14
Memory circuit
List of Abbreviations Used in Figures
AC
Anterior commissure
ACc
Anterior cingulate cortex
Ach
Acetylcholine
Ag
Amygdala
Al
Ansa lenticularis
Alat
Anterolateral nucleus (Thalamus)
Am
Anteromedial nucleus (Thalamus)
Ant
Anterior thalamus
Ap
Anterior perforate region
Ar
Arcuate nucleus (hypothalamus)
Av
Alveus
Bfb
Basal forebrain bundle
Bst
Bed of stria terminalis
CAg
Centromedial amygdala
Cc
Corpus callosum
Clau
Claustrum
Cd, h, t
Caudate nucleus, head, tale
Cg
Cingulate (gyrus)
Ci
Cingulum (longitudinal fascicle of the gyrus limbici)
Cao
Carrefour olfactif of Broca (paraolfactory area)
Cs
Cingulate sulcus
D1, 2
Dopamine receptor: types 1 and 2
DA
Dopamine neuron
DACg
Dorsal anterior cingulate
Db
Diagonal band of Broca
Dl
Dorsolateral nucleus (thalamus)
DLPF
Dorsolateral prefronal cortex
DMPF
Dorsomedial prefronal cortex
Dm
Dorsomedial nucleus (thalamus)
Dom
Dorsomedial nucleus (hypothalamus)
DPM
Dorsal premotor cortex
Ea
Extended amygdala
ECg
External cingulate gyrus
Ep
Epithalamus
Epl
Lateral habenula
Ent
Entorhinal cortex
Fa
Fascicle angularis
Fbc
Fronto-basal cortex
FEF
Frontal eye field
Fi
Fimbria
Fo
Fascicle olfactorius, diagonal band of Broca
FPc
Frontopolar cortex
Fr
Fascicle retroflexus
Fx
Fornix
Gaba
Gamma-aminobutyric acid neuron
Glu
Glutamate
gR
Gyrus rectus
Gp, e, i, v
Globus pallidum extern, intern, ventral
Hi
Hippocampus
Hy, l
Hypothalamus, lateral
Ical
Internal capsule anterior limb
Ida
Insular dysgranular area
Ifs
Inferior frontal gyrus
Ins
Insula
Ipn
Interpeduncular nucleus
Isth
Isthmus
Lat
Lateral nucleus (hypothalamus)
Lgb




Lateral geniculate body
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree