Chapter 4 Tissue repair
INTRODUCTION
Healing of all tissue is based on these phases and normally results in the formation of scar tissue. Limited regeneration of certain tissues, such as the epidermis, skeletal muscle and adipose tissue, can also occur. The basic principles that underlie repair and which lead to scar formation will be described first; subsequently, a brief summary of the regenerative healing of specific tissues is provided; finally, a number of factors that might be detrimental to repair are addressed.
THE PRINCIPLES OF TISSUE HEALING
The duration of each phase varies slightly depending on the type of wound, tissue involved and any complicating factors. Table 4.1 provides a general guide.
Table 4.1 Duration of and factors affecting repair phases
| Phase of repair | Approximate duration | Factors affecting duration |
|---|---|---|
| Inflammation | ||
| Acute phase | 24-48 hours | |
| Subacute phase | 10-14 days | |
| Proliferation | ||
| Variable intermediate phase | As above | |
| Remodelling | ||
| Begins at around 2 weeks; can last for a year or more | As above |
INFLAMMATORY PHASE
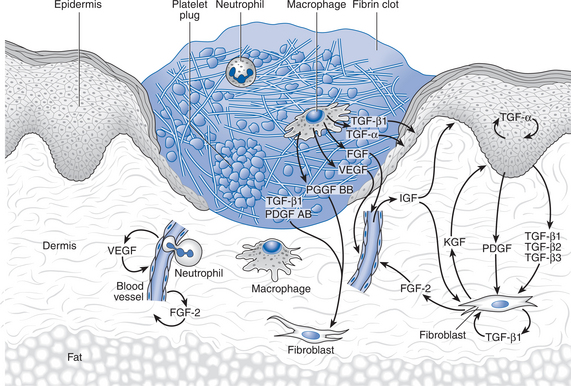
Tissue injury causes both cell death and blood vessel disruption. The primary purpose of the inflammatory phase of healing is to rid the area of debris and dead tissue and to destroy any invading infection prior to the repair. This phase can be described in terms of vascular and cellular changes, which are mediated through the actions of chemical agents. Figure 4.1 shows a typical wound after 3 days.
VASOREGULATION AND BLOOD CLOTTING
The initial vascular reaction involves haemorrhage and fluid loss due to destruction of vessels; this is followed by vasoconstriction, vessel plugging and blood coagulation, to prevent further blood loss. These processes lead to the activation of the repair process. Blood loss into the tissues initiates platelet activity and blood coagulation directly, both of which then result in the production of chemical factors which initiate and control the healing process. In addition, the blood clot provides a provisional matrix that facilitates the migration of cells into the wound (Clark 1991b, Singer & Clark 1999).
Both lymphatics and blood vessels are plugged to limit fluid loss. Initial platelet adhesion and aggregation is stimulated by the presence of thrombin (Terkeltaub & Ginsberg 1988). The platelets adhere to one another, to the vessel walls and to the interstitial extracellular matrix, leading to the build-up of relatively unstable platelet plugs. The process is continued and consolidated by the release of adhesive proteins such as fibrinogen, fibronectin, thrombospondin and von Willebrand factor by the platelets (Ginsberg et al 1988). Coagulation of extravascular blood is thought to be due to the action of platelets and intrinsic and extrinsic clotting mechanisms. Prothrombin is converted to thrombin and thus fibrinogen to fibrin, providing an early wound matrix.
Blood coagulation not only aids haemostasis through clot formation but adds to the early wound matrix and results in the generation of chemical mediators such as bradykinin (Proud & Kaplan 1988). These substances affect the local circulation, stimulate the production of further chemical mediators and act as attractants to cells such as neutrophils and monocytes.
Following this period of vasoconstriction, secondary vasodilation and increased permeability of venules occur owing to the effects of histamine, prostaglandins and hydrogen peroxide production (Issekutz 1981, Williams 1988). Subsequently, both bradykinin and the anaphyllatoxins initiate mechanisms that increase the permeability of undamaged vessels, leading to the release of plasma proteins which contribute to the generation of the extravascular clot.
CELL MIGRATION AND ACTION
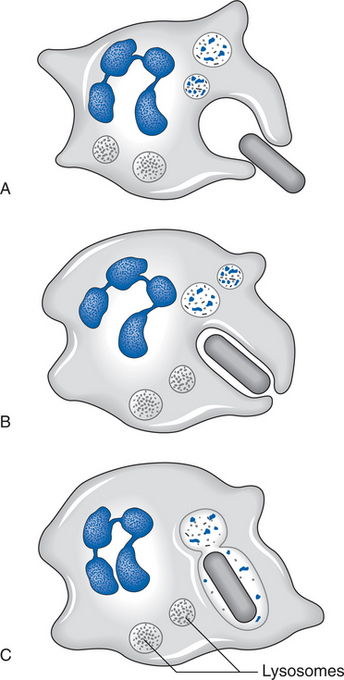
The primary action of neutrophils is phagocytosis; their task is to rid the site of bacteria and of dead and dying materials. Neutrophilic margination within the vascular structures leads to the passage of neutrophils through vessel walls by amoeboid action, enabling them to reach damaged extravascular tissues. Phagocytosis is achieved by neutrophilic lysis. This results in the release of protease and collagenase, which begin the lysis of necrotic protein and collagen respectively, as shown in Figure 4.2. Infiltration of the neutrophils into the extravascular tissue ends after a couple of days, marking the end of the early phase of inflammation.
Macrophages are essential to the healing process and can perform the normal function of neutrophils in addition to their other tasks. Monocytes migrate from the vasculature into the tissue space and rapidly differentiate into macrophages; the factors responsible for this change have not been fully identified, but may include the presence of insoluble fibronectin (Hosein et al 1985), low oxygen tension (Hunt 1987), chemotactic agents (Ho et al 1987) and bacterial lipopolysaccharides and interferons (Riches 1996). Macrophages phagocytose pathogenic organisms, tissue debris and dying cells (including neutrophils), and release collagenase and proteoglycans, both of which are degrading enzymes that lyse necrotic material (Leibovich & Ross 1975, Tsukamoto et al 1981).
CHEMICAL FACTORS
Many growth factors that influence and control the initial inflammatory process and trigger further developments in the proliferative phase are released by cells during the stage of inflammation; some key factors are shown in Table 4.2.
Table 4.2 Key cytokines affecting tissue repair (adapted from Singer ft Clark 1999)
| Cytokine groups | Arising from | Targets tissues and effects |
|---|---|---|
| Epidermal growth factors | ||
| EGF | Platelets | Epidermal and mesenchymal structures: |
| TGF-α | Macrophages, epidermal cells | cell motilityand proliferation |
| Heparin binding EGT | Macrophages | |
| Fibroblast growth factors | Macrophages, endothelial cells, fibroblasts | Wound vascularization, fibroblast proliferation, epidermal cell proliferation and motility |
| Transforming growth factor-β family | Platelets, macrophages | Fibrosis and increased tensile strength |
| Platelet-derived growth factor | Platelets, macrophages | Fibroblast proliferation and chemoattraction; macrophage chemoattraction and activation |
| lnterleukin-1 | Neutrophils | Production of growth factors |
| Insulin-like growth factor-1 | Fibroblasts, epidermal cells | Granulation tissue formation; re-epithelialisation |
| Tumour necrosis factor | Neutrophils | Production of growth factors |
| lnterferon-α/β | Lymphocytes, fibroblasts | Macrophage activation, inhibit fibroblast proliferation |
| Thromboxane A2 | Destroyed cells | Potent vasoconstrictor |
EGF, epidermal growth factors; EGT, epidermal growth factor; TGF, transforming growth factor.
Macrophages release factors that attract fibroblasts to the area (Tsukamoto et al 1981) and enhance collagen deposition (Clark 1985, Weeks 1972). Platelets release growth factors that contribute to the control of fibrin deposition, fibroplasia and angiogenesis through their action on a variety of cells. Platelets also release fibronectin, fibrinogen, thrombospondin and von Willebrand factor (Ginsberg et al 1988); these are necessary for the aggregation of platelets and for their binding to tissue structure. In addition, serotonin, adenosine diphosphate, calcium and thromboxin are released; these are necessary for blood vessel constriction to prevent haemorrhage.
Dead and dying cells release substances that influence the development of the neomatrix; these include a variety of tissue factors, lactic acid, lactate dehydrogenase, calcium, lysosomal enzymes and fibroblast growth factor (Clark 1991a). In addition, prostaglandins (PG) are produced by almost all cells of the body following damage, due to alterations in the phospholipid content of the cell walls (Janssen et al 1991); some types of PG are proinflammatory, increasing vascular permeability, sensitising pain receptors and attracting leucocytes to the area. Other classes of PG are anti-inflammatory. Both can be involved in early stages of repair.
PROLIFERATIVE PHASE
Fibroplasia
Fibroblasts produce and organize the major extracellular components of the granulation tissue. They migrate into the wound in response to both chemical and physical attractants (Clark 1990, McCarthy et al 1996, Repesh et al 1982). The fibroblast is primarily responsible for the deposition of the new matrix. Once present within the wound, fibroblasts synthesize hyaluronic acid, fibronectin and types I and III collagen; these form the early extracellular matrix. Changes take place as the matrix matures: the amount of hyaluronic acid and fibrinogen is gradually reduced, type I collagen becomes the predominant component, and proteoglycans are deposited.
Hyaluronic acid, present only in early wound healing, appears to facilitate cell motility and might be important in fibroblast proliferation (Lark et al 1985, Toole 1981). Fibronectin has many functions within a wound, including acting as a chemoattractant to cells such as fibroblasts and endothelial cells, augmenting the attachment of fibroblasts to fibrin, facilitating the migration of fibroblasts, and possibly providing a template for collagen deposition (Clark 1996). Proteoglycans contribute to tissue resilience and help to regulate cell motility and growth, and the deposition of collagen.
Collagen is a generic term covering a number of different types of glycoprotein found in the extracellular matrix. Collagen provides a rigid network, which facilitates further healing. The types of collagen within a wound, and their quantities, are gradually modified with time. Type III (embryonic collagen) is gradually absorbed and replaced by type I collagen, which is mature fibrillar collagen. Type IV collagen might be produced as a part of the basement membrane when skin damage occurs, and type V collagen is deposited around cells, forming a structural support.
Two primary factors affect collagen metabolism and, therefore, production. The first is the effect of the cytokines; Table 4.3 lists some of the cytokines believed to affect collagen metabolism. There appears to be a balance between the stimulatory and inhibitory effects of these substances, leading to optimal healing with neither over- nor underproduction of collagen.
Table 4.3 Cytokines controlling collagen production
| Cytokine | Action | Reference |
|---|---|---|
| TGF-β | Induces collagen synthesis | Ignotz & Massague 1986 |
| IL-1 | Induces collagen synthesis | Prostlethwaite et al 1988 |
| TNF | Induces collagen synthesis | Duncan & Berman 1989 |
| IFN | Decreases collagen synthesis | Czaja et al 1987 |
| TNF-α | Decreases collagen synthesis | Scharffetter et al 1989 |
| PGE2 | Decreases collagen synthesis | Nicholas et al 1991 |
IFN, interferons; IL-1, interleukin-1; PGE2, prostaglandin E2; TGF, transforming growth factor; TNF, tumour necrosis factor.
The second factor influencing collagen metabolism is the nature of the extracellular matrix (Kulozik et al 1991, Mauch & Krieg 1990). The extracellular matrix provides both a structural scaffold for the tissue and signalling for the cells. Reduced collagen synthesis results from cell contact with mature, type I collagen, upon which the production of collagenase is activated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree