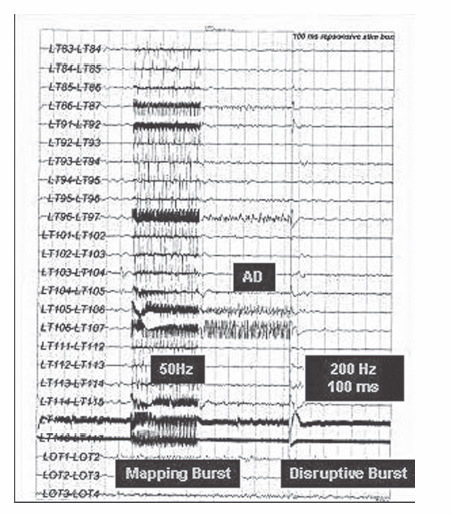
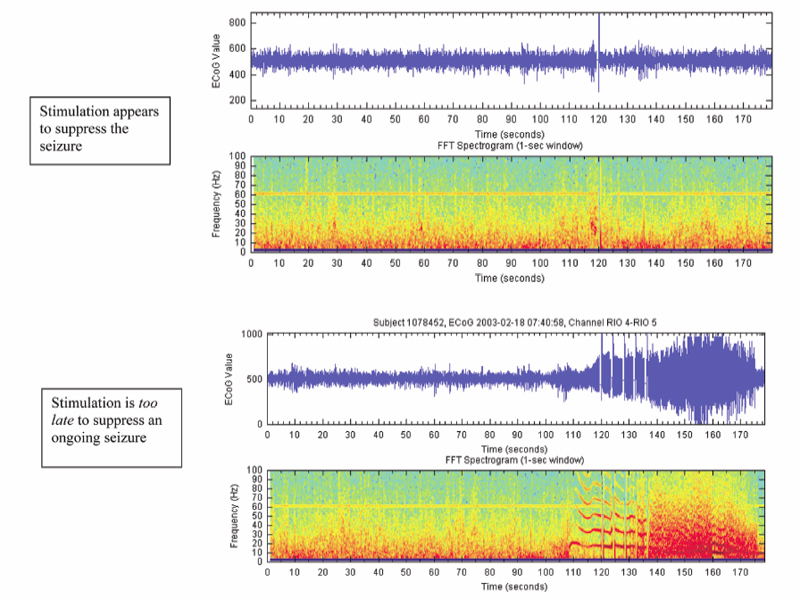
19 Responsive (Closed-Loop) Stimulation Partial epilepsy represents the most prevalent type of epilepsy, accounting for ~60% of cases overall and 90% of adult incident cases. Nearly 40% of these patients will be refractory to medications, resulting in more than 1 million patients in the US with medically refractory partial epilepsy.1 A few of these patients will be lucky (i.e., a 34-year-old female with intractable complex partial seizures, a right temporal cavernous angioma on magnetic resonance imaging [MRI], and an electroencephalogram [EEG] with right temporal sharp waves who has resective surgery and achieves an Engel class I outcome). Most patients, however, will not be so fortunate (i.e., a 29-year-old with disabling complex partial seizures, bilateral medial temporal sclerosis on MRI, an EEG with independent sharp waves and bilateral independent seizures, and ictal asystole observed in the epilepsy monitoring unit). For these medically refractory patients a few options are available such as vagal nerve stimulation, ketogenic diet, and experimental medications. Most will have continued seizures and side effects from medications. Given the significant need for improved therapeutics for patients with refractory seizures who are not candidates for resective surgery, there is considerable interest in device-based therapies. Vagal nerve stimulation, deep brain stimulation, and cortical stimulation are all being evaluated in clinical trials using open and closed-loop stimulation paradigms. The purpose of this chapter is to briefly review the use of neurostimulation for refractory epilepsy, and to describe in detail the use of responsive stimulation for the treatment of patients with medically refractory focal epilepsy using a cranially implanted responsive neurostimulator (RNS™ System, NeuroPace, Inc., Mountain View, CA). In 1870 two German physiologists, Gustav Fritch and Eduard Hitzig, examined localization of function by stimulating with electricity small regions of the exposed brain surfaces of awake dogs, providing evidence for localization of function in the cerebral cortex.2 The work of Fritsch and Hitzig was considerably expanded by Sir David Ferrier, whose work on cerebral localization was considered a great advance in the physiology of the nervous system. He stimulated the cortex of animals and defined precise areas related to movement, and later demonstrated the loss of motor function following resection of the corresponding areas. Ferrier boldly predicted, with good accuracy, the application of these findings to the human brain, and he assisted Macewen in the localization and resection of a cortical lesion in a patient with unilateral paralysis of the fingers and forearm.3 Penfield and Jasper greatly extended the use of electrical stimulation for localization of cortical function, and reported the first description of a seizure resulting from cortical stimulation.4 Initial trials of electrical stimulation for epilepsy treatment by Cooper and colleagues targeted the cerebellum.5–7 Although the subdural stimulation was well tolerated and was effective in small open trials, these results were not confirmed in later controlled trials8,9 A double-blind, randomized controlled pilot study of supermedial cerebellar cortex in five patients with motor seizures found a significant reduction in tonic-clonic and tonic seizures over 6 months of stimulation, but further surgery was required in four of these patients.10 Over the past 20 years a variety of brain targets in addition to the cerebellum (centromedian thalamus,11,12 anterior thalamus,13–15 subthalamic nucleus,16,17 hippocampus,18 posterior hypothalamus,19 and head of the caudate20) have been investigated for the capability of suppressing epileptic discharges using a variety of stimulation parameters. The potential use of brain stimulation to suppress seizures is based upon the premise that most forms of epilepsy result from an imbalance of excitatory and inhibitory processes due to debilitation of inhibitory processes,21 and the potential existence of a control system responsible for seizure arrest.22 The use of stimulation to artificially enhance the activity of such a control system can potentially suppress seizure activity. Experimental and clinical data have demonstrated that all of the above structures has some ability to exert inhibitory influences on both interictal and ictal epileptic electrical activity. Loddenkemper et al postulated a nigral control system capable of suppressing epilepsy via a dorsal midbrain anti-convulsant zone in the superior colliculi, and they suggested the potential interactions of the striatum, thalamus, and subthalamic nucleus, with the substantia nigra pars reticulata as the central part of the system that, when activated, inhibits seizure generation and propagation.17 Chronic anterior thalamic stimulation has been demonstrated in small, open-label clinical trials to reduce seizure frequency.13,14 A multicenter prospective randomized trial evaluating the safety and efficacy of chronic bilateral anterior thalamic stimulation in adults with medically intractable partial onset seizures with or without secondary generalization has completed recruitment (SANTE trial, sponsored by Medtronic, Inc.). Small, open-label trials have also reported seizure reduction in some patients treated with subthalamic stimulation.16,17,23 A randomized, double-blind, placebo-controlled, crossover assignment trial to assess the safety and efficacy of subthalamic nucleus stimulation for adults with medically refractory epilepsy is currently recruiting patients in France (www.clinicaltrials.gov). Another approach to the treatment of refractory epilepsy involves attempting to interfere with the ictal onset zone. Preliminary experience with trials in animals and humans is promising and suggests efficacy with both continuous (open-loop) stimulation and with responsive intermittent (closed-loop) stimulation. In addition, clinical data thus far provide reassuring safety data for responsive stimulation. Open-loop stimulation applied to mesial temporal structures reduced seizures in some patients with medically intractable temporal lobe epilepsy.18 Scheduled stimulation with hippocampal electrodes for 2 to 3 weeks prior to epilepsy surgery reduced the seizure frequency in 7 of 10 patients.24 An early clinical observation was that stimulation applied during functional cortical mapping was capable of disrupting induced afterdischarge potentials. The ability of applied cortical stimulation to successfully terminate spontaneous seizure activity in humans was explored in persons undergoing functional cortical mapping as part of an evaluation for epilepsy surgery. In several instances, a spontaneous electrographic seizure was recorded from the cortical electrodes. In some cases, delivery of a responsive stimulation (to the electrodes from which the electrographic event was recorded) appeared to stop the event (Fig. 19.1). Although this study was designed to explore safety and was not designed to demonstrate efficacy, one important observation in these early clinical evaluations was that stimulation was more likely to be effective if applied early in the electrographic event. Stimulation applied after electrographic seizure activity had become established did not appear to be effective in modifying or terminating the discharge (Fig. 19.2). Safety of responsive stimulation was assessed in an early clinical study using an External Responsive Neurostimulation System. This external, nonminiaturized version of the RNS™ System duplicated the automatic seizure detection and stimulation of the implantable system. The external system was applied to patients with implanted electrodes who had completed prolonged EEG monitoring for possible resective surgery. The External Responsive Neurostimulator System feasibility study was designed to evaluate the safety of responsive cortical stimulation with a secondary intent of gathering preliminary evidence (not statistical proof) of efficacy. Results of this study on 129 patients revealed no unanticipated serious device-related adverse events. The study also demonstrated that stimulation could be applied to multiple cortical areas without causing seizures, and that cortical stimulation at multiple frequencies and charge densities was well tolerated. Further observations were that stimulation applied early to an electrographic discharge could alter or terminate that discharge, that multiple electrodes in the vicinity of the seizure onset could be used for delivery of responsive stimulation, and that frequent responsive stimulation may be necessary to suppress clinical seizures. Fig. 19.1 Electrocorticography demonstrating that applied cortical stimulation disrupts afterdischarge potentials induced at surgery during functional cortical mapping. (Courtesy of Neuropace, Inc., Sunnyvale, CA. Reprinted with permission.) The RNS™ System consists of an implantable pulse generator and two implantable leads (subdural and/or depth leads) a programmer comprising a laptop computer with a wand and telemetry box; and a data transmitter that is given to the patient, consisting of a laptop computer with a wand and telemetry box (Figs. 19.3A and 19.3B). The neurostimulator continuously analyzes the patient’s electrocorticogram (ECoG) and automatically triggers electrical stimulation when specific ECoG characteristics programmed by the clinician are detected that represent either seizure or preseizure epileptiform activity (Fig. 19.4). In addition, the implantable neurostimulator stores diagnostic information including multichannel ECoGs and a detailed history of detections and stimulations. Using the data transmitter, the patient can upload this stored information to a secure Web site for analysis by the physician. Much of the technological basis of the RNS™ System builds on implanted defibrillator technology, in a sense creating an implantable brain defibrillator.
Localization of Cortical function
Deep Brain Stimulation for Epilepsy
Cortical Stimulation for Epilepsy
External Responsive Neurostimulation
The Implantable RNS™ System
System Components
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








