Chapter 20 Endovascular technology continues to advance at a dramatic rate. This is reflected in the complexity of vascular lesions treated and the decreasing number of aneurysms treated surgically. Today fewer aneurysms are uncoilable, unstentable, and unclippable. The use of revascularization techniques to treat these often complex lesions has resulted in high treatment success rates.1–5 What characteristics make an aneurysm untreatable? Which aneurysms are candidates for a revascularization procedure? Usually, the lesion is part of a main parent vessel. It may be defined based on its fusiform geometry, blister type, giant size, dissecting pathology, a calcified or atherosclerotic neck, a very broad or absent neck (dome to neck ratio < 1.5), a branch originating from the aneurysm, and an unstable intramural thrombus. Continued pathologic blood flow dynamics can exacerbate the pathology. Balloon and stent-assisted coiling can sometimes aid in securing these types of aneurysms; if not, then revascularization is considered.5–7 There are two main techniques used for revascularization. The first is an in situ bypass procedure such as side-to-side anastomosis, interposition grafting, patch grafting, and direct end-to-end resuturing. The second technique is the extracranial-intracranial (EC-IC) bypass. This includes low- and high-flow bypasses. Low-flow bypasses use external carotid artery (ECA) vasculature (superficial temporal artery [STA] and occipital artery [OA]). For high-flow bypasses, our group uses the radial artery (RA) and saphenous vein (SV) for grafting. Our patients are initially evaluated with computed tomography (CT), CT angiography (CTA), magnetic resonance imaging (MRI), and a six-vessel conventional angiogram. The CT scan can give insight into whether the aneurysm or parent vessel is calcified. The CTA gives anatomic detail of how the aneurysm relates to bony and brain structures. The MRI gives the best resolution of the brain surrounding the aneurysm. The MRI can also give additional information on the presence of thrombus within the lesion. We obtain a six-vessel angiogram to evaluate candidate vessels for the revascularization. The ECA and it branches are evaluated. Specifically, the STA and the OA are assessed for caliber and course. When the aneurysm involves the internal carotid artery (ICA) our group performs a 15-minute balloon test occlusion (BTO) with concurrent clinical examination. The BTO is also sometimes used when harvesting of the vertebral artery is anticipated. This test carries a risk of intimal dissection and/or thromboemboli. To decrease the risk, we use a low-pressure balloon and patients are placed on an aspirin regimen. For high-flow grafting, our group has had the most success with autologous grafts using the RA, and the SV as a second choice. We have limited experience using the tibial artery, when the RA and SV are not candidates. We routinely obtain ultrasound Doppler studies for mapping and sizing of the RAs. This includes an Allen test to document the patency of the palmar arch. Ultrasound studies are also used for mapping and sizing of the SVs and tibial artery if necessary. A multidisciplinary approach to the preoperative evaluation of the patient is used at our center.8–10 Patients who undergo a revascularization procedure to treat an aneurysm are also seen by a cardiologist and our neuroanesthesia team. A hematologic evaluation is also performed to exclude a coagulopathy. It cannot be stressed enough that the optimization of preoperative comorbidities is directly correlated with the patient’s long-term outcome. Once the patient and the lesion have been thoroughly evaluated, the treatment plan should be formulated by the whole team. This includes the neurosurgeon and his or her assistants, and the anesthesia, the monitoring, and the operating room staff. The choice of the bypass is based on the size of the vessel to be replaced. The graft should be roughly the same size or slightly larger than the vessel. The health and caliber of the donor and recipient vessels should be assessed carefully. The collateral circulation should also be weighed in on the procedure to be performed. The patient is given aspirin (325 mg/d) starting at least 1 week before surgery. Smokers are counseled on cessation. The patient is given perioperative antibiotics prior to the initial skin incision. An arterial blood pressure line is used for close monitoring. A central venous line is used to assess central venous pressure throughout the surgery to maintain an ideal fluid status. The patient is kept normocapnic and normotensive throughout the operation. We monitor motor evoked potentials (MEPs), sensory evoked potentials (SSEPs), and electroencephalogram (EEG) throughout the procedure. For surgery involving the posterior circulation, we also monitor brainstem function with SSEPs and brainstem auditory evoked responses (BAERs). The goal during temporary clip occlusion is to provide protection to brain at risk. This can be accomplished by decreasing the metabolism by the brain at risk. Both cooling and burst suppression are used. Cooling is investigational at this time: a randomized study is in progress at our center. In addition, by increasing the patient’s blood pressure, perfusion via collateral vascular supply can be optimized. At the time of temporary arterial occlusion, the patient is cooled (to 34°C), the systolic blood pressure is raised by 15 to 20% and EEG burst suppression is induced. Various anesthetic agents are used for burst suppression, but we tend to use propofol because it is relatively quick acting and reversible (compared with barbiturates). This reversibility also aids in a timely postoperative neurologic examination of the patient. For RA and SV grafts, during temporary occlusion, the patient is given 2000 to 2500 U of heparin for the prevention of thrombosis. This is not reversed at the end of the case. Prophylaxis for deep vein thromboses (DVTs) is begun on postoperative day #1. Examination and evaluation of the bypass graft and its anastomoses is performed with the use of an intraoperative micro Doppler and indocyanine green (ICG) angiography; these techniques combined have made the need for intra-operative angiography obsolete – they provide the surgeon and team valuable data on the patency and adequacy of the graft. If the integrity of the graft is at issue, it can be corrected prior to the conclusion of the operation. The STA can be harvested for interposition grafting (see below) or for STA-MCA bypasses. The STA caliber and location is ideal for anastomosis to the distal middle cerebral artery (MCA). This is extremely useful for distal MCA (M3 or M4) aneurysms. Occasionally, the need for an STA to superior cerebellar artery (SCA) graft emerges for the treatment of posterior circulation aneurysms. The preoperative angiogram should be evaluated to determine whether to use the frontal or the temporal-parietal branch of the STA. The STA is mapped to the skin with a Doppler probe. For aneurysm operations, we prefer a flap technique for dissecting the STA because this is easily combined with the craniotomy, and both branches of the STA can be extracted if necessary (Fig. 20.1). The artery is dissected distal to proximal. Under the microscope, the dissection is performed. One should leave closely adherent tissue to the vessel to avoid injury. Small branches can be bipolar cauterized distal to the vessel whereas larger branches can be microclipped or ligated. The vessel is left in situ until it is needed for the bypass. The distal anastomosis is sutured using 9–0 or 10–0 nylon sutures (Fig. 20.2). The heels of the graft are sutured with interrupted sutures. A single suture could be placed on the contralateral wall to prevent incorrect suturing of the edges. Running sutures, between anchored ends, are used because they are quicker than interrupted sutures. Prior to the last suture, the graft is flushed with heparinized saline to clear the air and test the anastomosis. Once the distal anastomosis is complete, the temporary clips are removed from the recipient vessel. The occipital artery can be used to treat posterior circulation aneurysms. It can be anastomosed to the anterior or posterior inferior cerebellar artery. It can also be anastomosed to the distal posterior cerebral artery (PCA; P3 or P4) or SCA. Occasionally, the vessel is long enough to use for MCA anastomoses when the STA is absent or small in caliber. This vessel should be localized with a Doppler probe and marked (Fig. 20.3). An inverted U-shaped incision, from the mastoid process to the midline, can be made and the flap reflected. It is found distally and traced proximally. The most difficult point of dissection is at the nuchal line, where it penetrates the muscle layers (along with the greater occipital nerve) to become more superficial. It is traced back to the digastric notch. The dissection of the OA is technically more difficult than the STA. In some patients the OA may be diseased and not exhibit good antegrade flow. In such cases, an interposition RA graft may be used instead. Fig. 20.1 Superficial temporal artery. Schematic showing the course of the frontal and temporal-parietal branches of the superficial temporal artery. The dashed line represents a linear incision near the vessel for a planned craniotomy at the shaded area. Alternatively, a “question mark” incision posterior to the dashed line will enable the harvesting of both vessels. (Copyright Laligam N. Sekhar.) Fig. 20.2 End-to-side anastomosis. (A) The end of the graft has been cut in a fishmouth shape. (B) Anchoring interrupted sutures on each end for orienting the anastomosis is important. (C) The medial wall of the anastomosis has been sutured with a continuous line. (D) The lateral wall can be sutured with interrupted sutures or can be sutured in a running fashion. (Copyright Laligam N. Sekhar.) These bypass grafts are used for the replacement of large caliber vessels (ICA, MCA, dominant vertebral artery, basilar artery). If the patient’s collateral circulation is poor, anastomosis with minimal occlusion of the parent vessel is of utmost importance. This is achieved with good planning and operative technique. The wall diameter is best matched to intracranial vessels, which makes this the vessel of choice in our group. The RA should be at least 0.23 cm in diameter by duplex scanning. A length of ~20 cm should be available. Suturing is easier than suturing of veins. An incision is begun just over the radial pulse at the wrist. The incision is made through the dermis without harming the artery. The artery is found and bluntly dissected from the surrounding subcutaneous tissue. Closely adherent fat and soft tissue is left on the artery. Branching veins to the venous plexus surrounding the artery are bipolar cauterized and sharply cut. The venae comitantes is preserved and only denuded from the RA graft at the ends. The branch arteries are microclipped distal to the RA and cut. The artery is harvested from the wrist to the brachial artery. It is left in situ until the bypass is performed. Prior to excision, the vessel is finger occluded to confirm the absence of change in the pulse oximetry on the finger. Once the RA is harvested, the periadventitial tissue is removed 1 cm near each site of anastomosis. This is done under the microscope to prevent damaging the endothelium of the vessel. Fig. 20.3 Occipital artery. This schematic illustration depicts the course of the artery. The shaded areas show an occipital and suboccipital craniotomy and the relationship to the artery. (Copyright Laligam N. Sekhar.) When the RA is not available the SV is used. These veins can be extremely difficult to extract in an obese patient. The SV should be at least 0.25 cm in diameter, for a length of 20 cm by duplex scanning. Suturing is more difficult than with the RA. Prior thrombosis or venous damage can make the vessel more prone to thrombosis. The valves within the walls of the vein can also put the patient at risk for thromboembolic events. Despite these drawbacks, it has been found that the patency rates of SV grafts are good. The annual failure rate is ~1 to 1.5%.11 An incision is made just medial to the palpable pulse of the femoral artery (Fig. 20.4). This extends as far as the adductor tubercle of the thigh. We mark the course of the vein by duplex scanning, which makes it easier to dissect from distal to proximal. The upper leg and the lower thigh are preferable because the vein has a more uniform caliber. The main vein is dissected from the subcutaneous tissue with blunt dissection. Surrounding fat that is intimately associated with the vein is left attached. Branches are clamped with microclips and sharply divided. These clips should be applied away from the graft so as not to harm the graft endothelium. The vein is left in situ until just before the bypass is performed. The exact length of the vessel for the graft should be measured with a suture (from proximal to distal anastomosis) and account for the tunneling. The graft should flow in the same direction, accounting for the intraluminal valves. We do not use endoscopic harvest because this has been shown to have a higher graft failure rate in the cardiac population.12
Revascularization Options for Complex Aneurysms
 Preoperative Evaluation and Preparation
Preoperative Evaluation and Preparation
 Anesthesia and Monitoring Considerations
Anesthesia and Monitoring Considerations
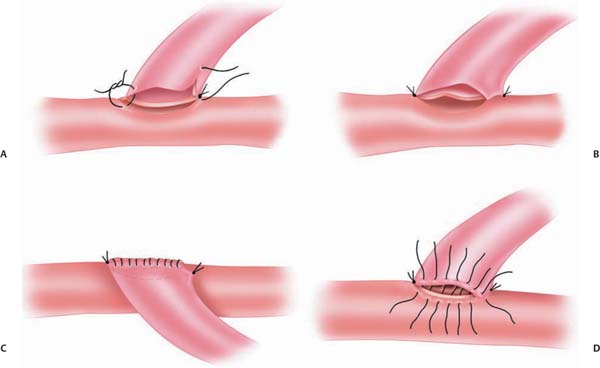
 Surgical Technique
Surgical Technique
Extracranial–Intracranial Bypasses
Low-flow Bypasses
Superficial Temporal Artery.
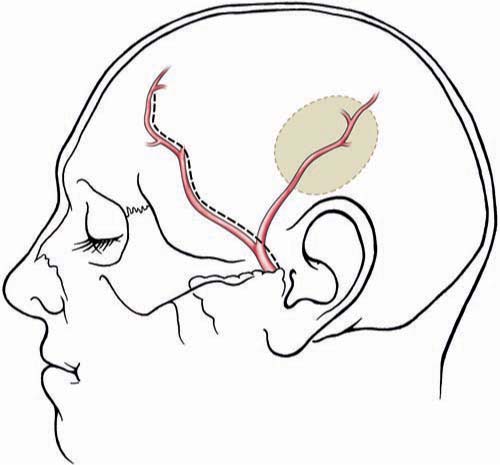
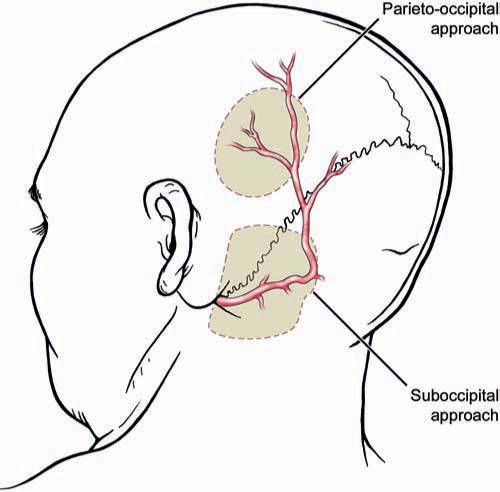
Occipital Artery.
High-flow Bypasses
Radial Artery Graft.
Saphenous Vein Graft.
In Situ Bypass Procedures
Side to Side
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree