12 | Rheumatoid Arthritis: Occiput–C1–C2 |
 | Case Presentation |
History and Physical Examination
The patient is a 70-year-old male with rheumatoid arthritis (RA). He had been followed for 12 years with serial imaging for a known diagnosis of dynamic atlantoaxial subluxation. His chief complaint was neck pain. Recently, the patient has also experienced occasional Lhermitte phenomenon with neck flexion. On physical examination, the patient had relatively preserved cervical range of motion. There was cervical pain in extremes of flexion and extension. Upper and lower extremity motor strength was normal. Upper extremity deep tendon reflexes were 3 + bilaterally with bilateral inversion of brachioradialis reflexes and positive Hoffmann sign. The patient ambulated with a bilateral, mildly coxalgic gait. Assessment of tandem gait was difficult due to hip and knee arthritis.
Radiological Findings
The anterior atlantodens interval (ADI) is zero in extension. In flexion, the ADI is 11 mm, and the posterior atlantodens interval (PADI) measures 12 mm (Fig. 12–1). The PADI in flexion had measured 21 mm 12 years prior and measured 16 mm 7 years prior. Magnetic resonance imaging does not demonstrate myelomalacia but does display moderate sub-axial canal stenosis (Fig. 12–2). The patient had subjectively preserved upper and lower extremity motor strength and sensation and was a community ambulator with the assistance of a cane for the last 4 years, primarily due to hip and knee arthritis. Other pertinent past medical history includes hypertension.
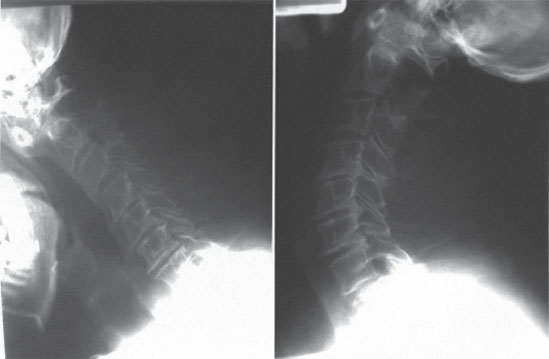
Figure 12–1 Lateral flexion-extension radiographs demonstrate dynamic atlantoaxial subluxation.
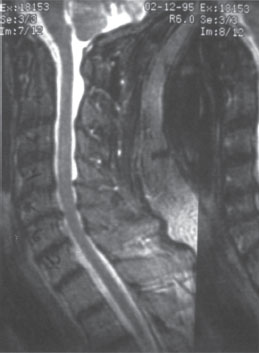
Figure 12–2 Magnetic resonance imaging demonstrates mild sub-axial stenosis and no myelomalacia. Note reduction of atlantoaxial subluxation in this supine imaging study.
Diagnosis
Rheumatoid atlantoaxial instability with risk for progressive myelopathy
 | Background |
Pathophysiology
The pathophysiology of RA is characterized by autoimmune synovial inflammation and pannus formation followed by destruction of articular cartilage, bone, and periarticular soft tissues. The C0-2 articulations depend heavily on soft tissue structures for stability and are a common site of spinal instability in RA. The two pathological entities commonly observed in this region in RA are basilar invagination (BI) and atlantoaxial instability (AAI). In BI, erosion of the lateral masses of C1 allows progressive settling of the cranium upon the cervical spine, sometimes resulting in brain stem compression by the dens. In AAI, instability develops in the horizontal plane, and the spinal cord may be compressed as the posterior arch of C1 subluxates anteriorly toward the dens. In both BI and AAI, hypertrophic pannus may contribute to neural compression. The end result of spinal cord or brainstem compression may be pain, neurological impairment, quadriplegia, or even death.
Osteoporosis is common in patients with RA, a result of both RA itself as well as from its treatment with corticosteroids. Significant osteoporosis compromises the surgeon’s ability to stabilize the spine with instrumentation. In recent years, the emergence of new disease-modifying antirheumatic drugs (DMARDs) has permitted better control of RA, and the number of patients presenting with severe cases of RA and spinal instability appears to be decreasing.
History and Physical Examination
Patients with BI or AAI may present with neck pain, extremity pain, or neurological impairment. Conversely, they may be asymptomatic; therefore, a high index of suspicion for cervical instability must be maintained by clinicians in cases of RA.
It is often difficult to elicit a complete history and physical examination (H&P) in patients with RA, but a thorough H&P is essential to provide the best care for the patient. Patients frequently present with a history of difficulty in ambulation, potentially due to RA involvement of lower extremity joints, steroid myopathy, or myelopathy. Motor strength of the upper and lower extremities is difficult to assess in the setting of joint and soft tissue destruction. Pertinent information includes presence or absence of neck pain, extremity pain, subjective extremity weakness, and its time course. A detailed timeline of the patient’s ambulatory capacity, use of assistive devices, and reasons for difficulty in walking (pain, weakness, shortness of breath, loss of balance, etc.) is essential.
Radiological Imaging and Diagnosis
The flexion-extension lateral radiograph is an excellent screening tool for BI and AAI. Both static and dynamic subluxations can be diagnosed. AAI is evaluated with the anterior ADI and the PADI. The normal ADI does not exceed 3.5 mm (5 mm in children). The PADI is a more direct measure of the space available for the spinal cord, and PADI of 13 mm or less has been correlated with spinal cord compression and myelopathy.1
Lateral radiographs provide good preliminary assessment of the occipitocervical junction, but do not provide visualization of soft tissue structures such as rheumatoid pannus or the spinal cord. Therefore, in patients with suspected cord compression, magnetic resonance imaging (MRI) or computed tomography with myelography (CTM) or both are indicated to provide definitive imaging of the spinal cord. Flexion-extension MRI and CTM may be helpful in cases of dynamic instability.
Numerous radiographic measurements have been devised to diagnose BI. Extension of the dens above McGregor line is a simple criterion for BI when bony landmarks are clear. The Ranawat index2 relies upon the anterior and posterior rings of C1 and the C2 pedicles as landmarks. These landmarks are usually visible even in the severely affected rheumatoid spine. Riew et al have shown that no single plain radiographic parameter has a high sensitivity and specificity for BI, and that using a combination of methods is more highly predictive.3 In cases where radiographs are suspicious for BI, MRI or CTM or both are indicated to evaluate the soft tissues and the neural elements. Bundschuh et al have shown that a cervicomedullary angle (angle between the cervical cord and the brainstem) of less than 135 degrees is predictive of neurological symptoms,4 but the role of the cervicomedullary angle in surgical decision making and prognosis has not been studied.
 | Methods of Management |
Treatments for cervical spine instability in RA include nonsurgical measures, such as observation or bracing, and surgical decompression and fusion. Clinical decision making in RA with spinal instability is challenging. The physician is charged with the responsibility of estimating the expected probability of progressive neurological injury with nonsurgical treatment, the expected benefit to the patient from a successful surgical intervention, as well as the potential complications that could result from surgery. Indications for surgery include progressive neurological injury with radiographic spinal cord or brainstem compression, intractable pain, or significant neural compression with or without neurological injury.
Surgical intervention in rheumatoid cervical instability consists of decompression and fusion. Posterior decompression via laminectomy is the simplest and safest means of decompression in the upper cervical spine. In rare cases where posterior surgery alone does not provide adequate decompression of neural structures, anterior odontoid resection may be performed via transoral5 or high retropharyngeal6 approaches. In the setting of a solid posterior arthrodesis, reduction in the size of rheumatoid pannus is nearly universal7 and may obviate the need for direct anterior decompression.
External orthoses and halo vests can provide supplemental stabilization of the C0-2 junction.8 Soft collars are not effective in limiting motion in any plane. Atlantoaxial motion is reduced ~50% by rigid collars (Miami J Collar, Jerome Medical, Moorestown, NJ, or Aspen Cervical Collar, Aspen Medical Products, Irvine, CA) and 75% by a halo vest. Halo vests provide the best occipitocervical immobilization but are associated with numerous complications9 and are poorly tolerated by the elderly.10 Fortunately, the development of more stable instrumentation techniques has reduced the need for postoperative halo vest immobilization.
Atlantoaxial Fusion
There are numerous techniques for instrumentation of the upper cervical spine. The techniques of Brooks and Jenkins11 and Gallie12 utilize sublaminar wires and structural iliac autograft for atlantoaxial fusion. Wiring techniques have the advantage of requiring inexpensive and simple equipment (wire or cable) that is readily available in most operating rooms. On the other hand, intact laminae are required for fixation, and there is some risk to the passage of sublaminar wires. Finally, the stability imparted by wire constructs is limited, particularly in axial rotation and lateral bending. This results in limited ability to maintain precise reduction of vertebrae as well as relatively higher rates of pseudarthrosis when compared with more modern instrumentation techniques.
Alternatives to wiring have been developed. The Halifax clamp system utilizes laminar hooks for C1-2 fixation, resulting in good control of flexion moments but less restriction of extension, lateral bending, and axial rotation. Stability of the Halifax clamp construct is enhanced by placement of a structural graft to block extension. Intact laminae are required. The Halifax system is currently not widely used, probably because significant nonunion rates have been reported.13
The Magerl technique for placement of transarticular screws provides excellent fixation of C1-2,14 particularly when used in combination with sublaminar wiring. Intact laminae are not required. There are several limitations to the technique. Obtaining the correct trajectory for screw placement may require exposure of several levels caudal to C2. The C1-2 joints must be sufficiently reducible for screw placement. Finally, there is a risk of vertebral artery injury, particularly in cases of aberrant or dilated vertebral artery anatomy. Sagittal CT reconstructions should be carefully scrutinized to ensure that the C2 isthmus is large enough to accommodate a screw.15
Harms and Melcher have described a technique for C1-2 stabilization using screws in the C2 pedicle and C1 lateral mass.16 Both Magerl and Harms techniques are technically challenging but result in high fusion rates. In contrast to the Magerl technique, the techniques of Harms and Melcher can be used in patients with irreducible atlantoaxial subluxation.
Occipitocervical Fusion
Stabilization of the occipitocervical junction poses unique challenges. The C0-2 articulations provide a large range of motion in multiple planes. Occipitocervical instrumentation is exposed to large loads because the occiput is relatively massive and possesses a long lever arm. A wiring technique for occipitocervical fusion was described by Wertheim and Bohlman.2 This technique employs wires passed through drill holes in the external occipital protuberance, a sublaminar wire at C1, and a spinous process wire at C2. Massive iliac autografts serve a structural and biological function and are secured by the wires and span the posterior surfaces of the occiput and C1-2 laminae. Fusion rates of 85 to 100% have been reported.2,17 Wiring techniques are technically simple and do not require highly specialized equipment or implants. On the other hand, supplemental external stabilization with a halo vest or four-poster cervical collar is usually necessary, laminar arches must be intact, and rigid fixation is generally not achieved.
Rigid metallic loops or rectangles, initially developed for use in the thoracolumbar spine, have been adapted for occipitocervical use. The Luque rectangle exemplifies this class of implants. Rigid loops are secured to the spine and occiput using combinations of sublaminar, spinous process, and transcranial wires. These constructs provide greater stability than simpler wiring methods and result in high fusion rates (~90%) and reduced need for halo vest immobilization.18–20 Limitations of this class of implants include relatively high profile leading to implant prominence, need for intact laminae and sublaminar wire passage, and limited ability to correct and maintain reduction of deformities. Reduction of BI, in particular, is not well maintained by wiring techniques or rigid loops.21
Constructs utilizing screws connected by rods or plates represent the most recent development in occipitocervical instrumentation. These constructs achieve fixation via screws placed into the occiput, cervical lateral mass, and cervical pedicle. Fixation of C1 can be achieved with Magerl C1-2 transarticular screws or C1 lateral mass screws. The stability of screw-based constructs is superior to that of wire constructs,22 allowing better reduction of deformity and high fusion rates without the use of halo vest immobilization.23,24 Decompressive laminectomy does not compromise fixation as when using wiring techniques. On the other hand, occipitocervical screw placement is technically challenging and poses the risk of injury to the spinal cord, nerve roots, cerebellum, dura, and vertebral artery.
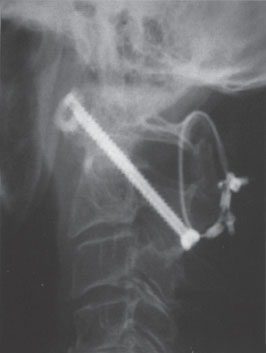
Figure 12–3 Postoperative lateral radiograph.



