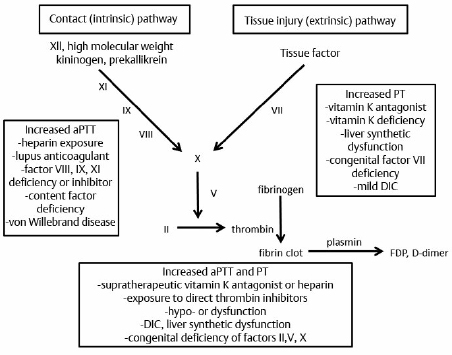
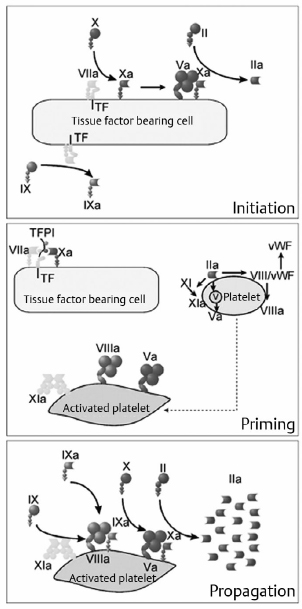
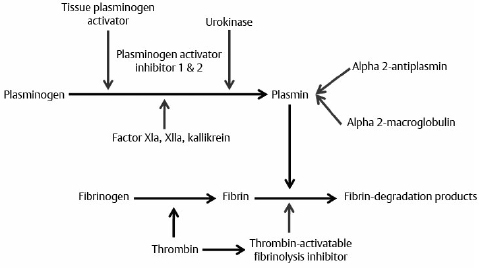
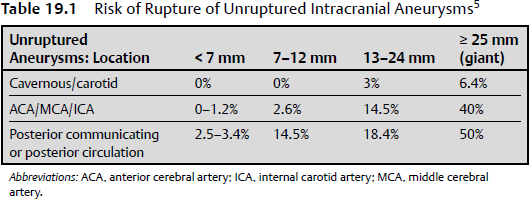
19 There is a paucity of high-level evidence (lack of prospective studies) investigating the risks of anticoagulants and antiplatelet agents in patients with common neurovascular conditions. In view of the current state of evidence, clinical decisions regarding initiating or maintaining a patient on a specific antiplatelet or anticoagulant drug depend on the balance of the following risks: 1. Risk of bleeding with each type of vascular lesion or malformation 2. Risk of intracranial hemorrhage with each type of anticoagulant/antiplatelet agent 3. Additive risk of increased bleeding with vascular malformation and anticoagulant/antiplatelet (from disruption of thrombotic clot) 4. Risk of an event (e.g., cardioembolic stroke from atrial fibrillation) for which an anticoagulant/antiplatelet agent is initiated 5. Risk reduction of an event after initiation of an agent Only level 3 evidence (evidence from case series, historical controls, case reports, and expert opinion) is available regarding risk management, leading to high degrees of clinical uncertainty. The initial response to vessel injury begins with formation of the platelet plug, which depends on normal vascular and platelet function. Defects in one or a combination of these components are reflected in a prolonged bleeding time. Bleeding disorders may be secondary to defects in the activity of platelets, blood vessel endothelium, or one or more coagulation factors (coagulopathy). The causes may be congenital or acquired. This chapter focuses on acquired causes of defects in platelet activity, such as medications, renal diseases, myelodysplasia, and myeloproliferative disorders, and on acquired defects in coagulation, such as exposure to anticoagulants, vitamin K deficiency, liver disease, disseminated intravascular coagulopathy (DIC), trauma, and acquired factor inhibitors. The classic representation of the coagulation cascade includes an extrinsic pathway initiated by tissue injury and a contact (intrinsic) pathway, leading to the common pathway (Fig. 19.1). This system is useful for understanding causes of prolonged activated partial thromboplastin time, which include heparin, lupus anticoagulant, deficiencies of factors VIII, IX, and XI, and von Willebrand disease. Elevated prothrombin time may be due to warfarin, vitamin K deficiency, liver dysfunction, congenital factor VII deficiency, and mild DIC. Thrombin time depends on the final step of the coagulation cascade (conversion of fibrinogen to fibrin). Therefore, this test is sensitive to detection of fibrinogen abnormalities and inhibitors acting at this level (for example, heparin). Fig. 19.1 Classic view of coagulation cascade showing extrinsic and intrinsic pathways. aPTT, activated partial thromboplastin time; DIC, disseminated intravascular coagulation; FDP, fibrin degradation products; PT, prothrombin time. Current understanding of coagulation, however, is that in vivo it is a three-step process centered on cell surfaces (Fig. 19.2).1,2 Multiple mechanisms exist to localize coagulation to the site of injury. Factors VII and VIIa have little enzymatic activity unless tissue factor is present and there is very little circulating tissue factor. Amplification requires binding of platelets to the site. Inhibitors of coagulation, such as antithrombin 3, inactivate any circulating factor Xa and thrombin (factor II). Thrombin also binds thrombomodulin on adjacent undamaged vessels, which activates protein C and, in a cascade, protein S, which in turn inactivate factors Va and VIIIa. Circulating tissue factor pathway inhibitor also inhibits the tissue factor—factor VIIa complex. Fibrin clot must be maintained for long enough to permit repair of vascular injury, or delayed bleeding may occur. Fibrinolysis, however, is initiated under various circumstances by activation of plasminogens (tissue and urokinase types) to plasmin, which degrades fibrin to fibrin degradation products (Fig. 19.3).3 Fibrinolysis can be detected by measurement of fibrin degradation products and D-dimer. D-dimer is a nonspecific product of plasmin degradation of fibrin. Normal inhibitors of the conversion of plasminogen to plasmin are plasminogen activator inhibitor (PAI-1), and thrombin-activatable fibrinolysis inhibitor (TAFI, mainly α2-antiplasmin). Currently available inhibitors of fibrinolysis include tranexamic acid and aminocaproic acid. Fig. 19.2 Drawing of the three phases of cell-based coagulation.1 Coagulation begins primarily by initiation with tissue factor (TF), which is present on the subendothelium, tissues not normally exposed to blood, activated monocytes, and endothelium when activated by inflammation.2 Factors VII and VIIa bind to tissue factor and adjacent collagen. The factor VIIa tissue factor complex activates factor X and IX. Factor Xa activates factor V, forming a prothrombinase complex (factor Xa, Va, and calcium) on the tissue factor expressing cell. Coagulation is amplified as platelets (Pl) adhere to the site of injury in the blood vessel. Thrombin is activated by platelet adherence and acts then to fully activate platelets, enhance their adhesion, and release factor V from the platelet α granules. Thrombin on the surface of activated platelets activates factors V, VIII, and XI, with subsequent activation of factor IX. The tenase complex (factors IXa, VIIIa, and calcium) are now present on platelets where factor Xa can be produced and generate another prothrombinase complex on the platelet so that there can be large-scale production of thrombin. Propagation is the third stage and is a combination of activation of the prothrombinase complexes that allow large amounts of thrombin to be generated from prothrombin. More platelets can be recruited, as well as activation of fibrin polymers and factor XIII. Incidental intracranial aneurysms are present in 2% of adults.4 Several studies have estimated the risk of rupture of unruptured intracranial aneurysms.5,6 These may be found in patients who have multiple intracranial aneurysms presenting with subarachnoid hemorrhage (SAH) from one of the aneurysms, when an aneurysm becomes symptomatic but not ruptured, or incidentally. Wermer and colleagues7 reported a meta-analysis of 19 studies including 4,705 patients with 6,556 unruptured intracranial aneurysms followed for 26,122 patient-years. The risk of rupture was 1.2% per patient year in the first 5 years of follow-up, 0.6% per year between 5 and 10 years, and 1.3% beyond 10 years. The risk of rupture was significantly higher in patients over 60 years of age, females, Japanese or Finnish patients, those with larger aneurysms or posterior circulation aneurysms, or if the aneurysm was symptomatic but unruptured. Smoking was associated with increased risk of rupture but was not significant. Growth of aneurysms is believed to be a risk factor for rupture, but may not be detected in natural history studies because these patients are treated and removed from analysis. The largest study of unruptured aneurysms included 1,692 patients with 2,686 aneurysms followed for a mean of 4.1 years.5 Cumulative 5-year risk of rupture was lower than the numbers above and was increased in patients with a prior SAH, an aneurysm of larger size, and an aneurysm located in the posterior circulation. In Japan, Ishibashi et al6 followed 419 patients with 529 unruptured aneurysms for a mean of 2.5 years. Nineteen aneurysms ruptured, for a 1.4% rupture rate per year. Risk factors for rupture were increasing size, a history of SAHs, and posterior circulation aneurysm (Table 19.1). Other risk factors for rupture that are less well documented include various morphological characteristics of the aneurysm (daughter loculi, irregular shape, high aspect ratio), growth of the aneurysm on serial imaging, preexisting hypertension, and family history of intracranial aneurysms.8 The risk of morbidity and mortality from aneurysm rupture also is important to consider. Mortality in studies of unruptured aneurysms that then rupture ranged from 42 to 65%.5,6,9 Furthermore, at least 50% of survivors have permanent cognitive and neurologic impairment.10 Patients with unruptured aneurysms frequently harbor risk factors for ischemic heart and cerebrovascular disease and are candidates for treatment with antiplatelet drugs. The beneficial effects of antiplatelet drugs, particularly acetylsalicylic acid, have been reviewed. There is class 1, level A evidence to support the use of antiplatelet drugs (acetylsalicylic acid, acetylsalicylic acid plus dipyridamole, or clopidogrel) for secondary prevention of recurrent stroke and cardiovascular events in patients with noncardioembolic ischemic stroke or transient ischemic attack,11 including those with atrial fibrillation who cannot take anticoagulants.12 The relative risk reduction for any type of stroke (ischemic or hemorrhagic) is 15%, and that for overall vascular death and disease is 20%.13 Compared with placebo, acetylsalicylic acid (aspirin) decreased vascular events (and their morbidity and mortality) in patients with coronary, cerebrovascular or peripheral vascular disease (odds ratio [OR] = 0.71, 95% confidence interval [CI], 0.67–0.76; OR = 0.87, 95% CI, 0.82–0.93; and OR = 0.50, 95% CI, 0.29–0.88, respectively).13,14 The risk of major hemorrhage, on the other hand, was almost doubled by aspirin (OR = 1.87, 95% CI, 1.51–2.32 for all three indications).14 These numbers grossly translate, depending on the indication and events prevented, to roughly 20 to 40 vascular events and deaths prevented when 1,000 patients are treated with antiplatelet therapy for about 2 years, and to less than five major hemorrhages. The question arises as to whether antiplatelet drugs increase the risk of aneurysm rupture, and, in the event of rupture, if the outcome is worse. There is no high-level evidence that antiplatelet drugs increase the risk of aneurysm rupture, or that the outcome is worse if the aneurysm ruptures. Given the shared risk factors (smoking, hypertension, age, among others) and the prevalence of unruptured aneurysms in the general population, it is likely that there are a substantial number of patients currently on antiplatelet drugs with undiagnosed, unruptured aneurysms.15 Several small series report patients presenting with ischemic stroke who are found to have aneurysms.15 In one series, there were no ruptures among 16 patients with ischemic stroke and unruptured aneurysms who were followed for 3 months on antiplatelet drugs.15 There were 90 (3%) of 2,885 patients in the North American Symptomatic Carotid Endarterectomy Trial with unruptured intracranial aneurysms.16 Eighty-two were followed for a mean of 5 years. One patient died of myocardial infarction 6 days after carotid endarterectomy, and the autopsy showed an SAH, but there was no evidence that the aneurysm was the source. Best medical management in these patients included aspirin, so it is likely that this is a cohort of patients with unruptured aneurysms who were on antiplatelet drugs for years with no SAH.17 Numerous studies examined acetylsalicylic acid use in patients admitted to the hospital with an SAH. One study of 305 patients admitted with an SAH found that 29 gave a history of acetylsalicylic acid use.18 There was no difference in outcomes between the two groups, suggesting that treatment of patients with unruptured aneurysms who then had an SAH would be no different from those not on acetylsalicylic acid. Limitations of the data included the lack of information on patients who died without being admitted to the hospital. The authors opined on the use of antiplatelet drugs in patients with unruptured aneurysms and could offer no compelling evidence against their use if medically indicated. The risk of stroke, other morbidity, and death without antiplatelet drugs needs to be considered, but it is likely that the balance will be in favor of treating many patients with unruptured aneurysms with antiplatelet drugs when they are indicated for medical reasons. Many people take acetylsalicylic acid and other nonsteroidal anti-inflammatory drugs for minor health problems or for perceived beneficial effects on vascular disease and stroke when no benefit exists. Patients with unruptured aneurysms probably should be advised to avoid these drugs unless there is a specific medical indication for them. Several studies examined the efficacy of acetylsalicylic acid and other antiplatelet drugs for preventing delayed ischemia and improving outcome in patients with an SAH.19–21 Meta-analysis of the first five randomized, placebo-controlled trials found that the overall risk of poor outcome was 0.87 (95% CI, 0.65–1.17), and of delayed cerebral ischemia (DCI, reported in three of the five studies), 0.65 (95% CI, 0.47–0.89). The drugs used included acetylsalicylic acid, the thromboxane synthetase inhibitor OKY-046, and dipyridamole. This finding suggested that a larger randomized trial was warranted, but that study was stopped early when the chances of detecting a beneficial effect were statistically negligible.20 The question arises as to whether to stop antiplatelet drugs when a patient is admitted with an SAH. In general, this is done, given the lack of obvious clinical benefit for being on these drugs after an SAH and the lack of any documented prothrombotic state when stopping them. Anticoagulants, such as warfarin, increase the risk of fatal or disabling intracranial hemorrhage 1.5 times22; intracranial hemorrhage causes 90% of warfarin-related death.23 The risk of intracranial hemorrhage while on anticoagulants is 0.3 to 1% per year and is increased compared with the untreated population by 0.2% per year.24 The outcome from anticoagulant-associated intracerebral hemorrhage (ICH) is worse than after spontaneous ICH.25 Mortality was 33% within 1 day compared with 16% in those not taking anticoagulants, and 66% versus 50% at 1 year.25 A study of 1,188 hospital admissions for SAH compared with 11,880 controls found no association between the risk of an SAH and anticoagulants. The study did not include deaths before admission, so it is still possible that anticoagulation increases the risk of a fatal SAH. Rinkel et al26 identified 15 patients admitted to the hospital with an aneurysmal SAH who had been taking anticoagulants. The relative risk of poor outcome was 1.9 times higher compared with 126 control patients with an aneurysmal SAH.26 The study excluded patients on anticoagulants who had an international normalized ratio (INR) < 1.5. Thus, anticoagulants, when administered at a dose that is therapeutic, worsen the outcome from an SAH, but whether they increase the risk of it occurring is less clear and not documented in the literature. If anticoagulation is indicated in a patient with an unruptured aneurysm, this information should be taken into account because, depending on the risk of ischemic stroke if the patient were not on anticoagulants, the balance might tip in favor of treating the unruptured aneurysm before initiating anticoagulation. Decisions will be individualized in these cases because many of these patients are elderly and have factors that increase the risk of treatment of the aneurysm. There also is no information to inform decisions about how to manage patients with unruptured aneurysms who require prophylactic anticoagulation, for example, when undergoing other surgical procedures like joint replacements. Although the risk of anticoagulant-associated ICH is higher when anticoagulants are first started, the duration of treatment in such cases is shorter. If a patient with an unruptured aneurysm presents with an SAH, then rapid reversal of anticoagulation to achieve normal coagulation is indicated. The guidelines for reversal of anticoagulation in patients with anticoagulation-associated ICH are reasonable to use given the lack of any other information.27 Occasionally, a patient with an unruptured aneurysm may present with acute ischemic stroke and may be a candidate for thrombolytic therapy. Thrombolytic therapy increases the risk of fatal intracranial hemorrhage threefold, or 7% of patients treated, but improves the chances of a good outcome to a greater degree when administered within 4.5 hours of the onset of stroke in appropriate patients.28 Another relevant statistic is that intracranial hemorrhage occurs in 0.3 to 1% of patients treated with thrombolytics for myocardial infarction.29 A review of the literature up to 2004 found seven cases of patients with unruptured aneurysms treated with intraarterial or intravenous urokinase or tissue plasminogen activator. One aneurysm ruptured (14%) and the patient died. The authors concluded that therapeutic decision making in this situation was difficult, and that physicians may often avoid using thrombolytic therapy in patients with known aneurysms, although the evidence to support this practice is lacking, and it is possible that patients with small or treated aneurysms are candidates for treatment. Arteriovenous malformations (AVMs) are responsible for 1 to 2% of all ICHs and have an annual risk of hemorrhage of 2 to 4%.30,31 The outcome after a first hemorrhage is better than after an aneurysmal SAH or spontaneous primary ICH, with death occurring in 10% and permanent morbidity in 30%.31,32 Rebleeding is believed to be more common in the year after hemorrhage, occurring in 6% of cases and then declining to a baseline of 2 to 4%. Emerging evidence, however, suggests the risk of hemorrhage depends on a variety of factors (Table 19.2). Multivariable analysis of 622 consecutive patients with AVMs from a prospective database found that hemorrhage was associated with increasing age, initial presentation with hemorrhage, deep location of the AVM, and exclusive deep venous drainage.33 The risk of hemorrhage varied from 0.9% per year for patients with no history of hemorrhage and a non-deep location with no exclusive deep venous drainage, to 34% for patients with all three risk factors. Da Costa et al34 studied a prospective cohort of 678 patients with AVMs. The annual risk of hemorrhage overall was 4.6%, compared with 7.5% for those presenting with hemorrhage, 4% for those presenting with seizures, 4% for those with no aneurysms, and 6.9% for those with aneurysms. Presentation with hemorrhage was significantly associated with the future risk of hemorrhage, and associated aneurysm or deep venous drainage showed trends toward this association. Other studies have suggested an increased risk of hemorrhage in patients with single draining veins, venous outflow stenosis, hypertension, and associated aneurysms. AVMs are associated with three types of aneurysms. There are flow-related aneurysms on arteries supplying the AVM in 11%, intranidal aneurysms in 6%, and aneurysms located on arteries not supplying the AVM in 1%.35 Table 19.2 Annual Rates of Hemorrhage for Subtypes of Arteriovenous Malformations
Risk of Anticoagulants and Antiplatelet Agents in Common Neurovascular Conditions
Coagulation Cascade and Acquired Defects
Vascular Lesions
Unruptured Intracranial Aneurysms
Antiplatelet Drugs and Aneurysms
Anticoagulants and Aneurysms
Unruptured Arteriovenous Malformations
| No Deep Venous Drainage | Deep Venous Drainage |
Nidus, not deep | 1% | 3% |
Nidus, deep | 3% | 8% |
There is a lack of epidemiological studies investigating anticoagulant and antiplatelet use, and bleeding rates in patients with AVMs. In general, we recommend that they avoid taking these drugs. Again, this has to be balanced against the indication for the drug, the risk of stroke, vascular events, and death if the drug is not taken, and the risk reduction achieved if the drug is taken. The most common situation would be the elderly patient in whom antiplatelet drugs are indicated for vascular disease. It is likely that in most cases the risks and benefits would favor treating such patients with these drugs if they had an untreated or untreatable AVM.
As with other types of intracranial hemorrhage secondary to vascular lesions, if a patient with an AVM presents with a hemorrhage while on anticoagulants, then rapid reversal of anticoagulation to achieve normal coagulation is indicated. The guidelines for reversal of anticoagulation in patients with anticoagulation-associated ICH are reasonable to use given the lack of any other information.27
Cavernous Malformations
Cavernous malformations (CMs) are low-flow, low-pressure vascular malformations characterized pathologically by thin-walled vascular channels with collagenous walls and endothelial cell linings. The channels are of varying size and contain blood or in some areas thrombus of varying degrees of organization. There is no intervening brain tissue and the brain around the lesion usually shows gliosis and contains hemosiderin-laden macrophages. They are found in 0.5% of adults.36 Genetic linkage studies have identified three loci for CMs; CCM1 (Krev interaction trapped 1 [KRIT1]) on 7q21–22, CCM2 (MGC4607 or malcaverin) on 7p13–15, and CCM3 (programmed cell death 10 [PDCD10]) on 3q25.2–27.37 About 75% of familial cases have identified abnormalities in these genes, whereas mutations are identified in fewer sporadic cases. Fifty percent to 75% percent of patients with familial CM have multiple lesions compared with 15% of patients with sporadic CM.38 The annual risk of clinically significant first hemorrhage from CM is 0.1% to 2.7% per lesion.38 The annual risk of rebleeding after the first episode of hemorrhage is 4.5%, and there may be clustering of hemorrhages interspersed with periods of quiescence. An increased risk of hemorrhage has been associated with prior hemorrhage, deep or brainstem location, and possibly female sex.
As with AVMs, there are no epidemiological studies investigating anticoagulant and antiplatelet use and bleeding rate in patients with CMs. We found one case report of a 42-year-old woman with familial CM who developed hemorrhage during treatment with prophylactic low molecular weight heparin after undergoing a hysterectomy.39 She survived and did not suffer permanent morbidity. The authors cautioned against drawing any conclusions about the relative risks of anticoagulant drugs in patients with CM. We recommend advising patients with CMs to avoid taking anticoagulant or antiplatelet drugs unless there is a specific medical indication, in which case the risks and benefits should be reviewed and assessed on a case-by-case basis. The low risk of hemorrhage and of morbidity and mortality from hemorrhage from CMs is an important consideration in such decisions.
If a patient presents with symptomatic hemorrhage from a CM, rapid reversal of anticoagulation to achieve normal coagulation is indicated. The guidelines for reversal of anticoagulation in patients with anticoagulation-associated ICH are reasonable to use given the lack of any other information.27
Intracerebral Hemorrhage
Chronic hypertension causes 50 to 70% of ICHs. Cerebral amyloid angiopathy is believed to be the cause in 10% of all cases and in up to 30% of lobar hemorrhages in the elderly. Rupture of vascular malformations is the etiology in 5 to 13% and is more common in younger patients. Congenital or acquired coagulopathies, the latter most commonly due to anticoagulant drugs, are the fourth most common cause, accounting for 5 to 6% of all cases of ICH. Recurrence of ICH has been associated with lobar location, increased age, use of anticoagulants, apolipoprotein E types 2 and 4, alleles, and microhemorrhages on T2*-weighted gradient echo magnetic resonance imaging (MRI).27 Anticoagulant-related ICH is more likely with increasing age, prior ischemic stroke, hypertension, leukoaraiosis (also known as nonspecific white matter changes), within the first months of starting anticoagulants, and if the INR is higher.24
The emergency management of patients taking anticoagulants who present with ICH includes reversing anticoagulation as quickly as possible with prothrombin complex concentrates or fresh frozen plasma and intravenous vitamin K. In patients with ICH who are taking antiplatelet drugs, platelet transfusions were considered investigational.27 Hematoma growth is noted in up to 38% of patients with ICH when computed tomography (CT) scans can be compared within 1 hour and then within 3 hours of onset.40 Growth is observed in 16% of those who are first imaged between 3 and 6 hours, and 10 to 15% show hematoma enlargement between 6 and 24 hours from onset of ICH.41 Risk factors for hematoma growth include early presentation, a spot sign on CT scan, larger initial ICH, use of anticoagulants, presence of intraventricular hemorrhage, and reduced platelet activity.42,43
There are two questions that arise concerning the patient with ICH: whether to start or resume anticoagulation, and if so, when.
Whether to Start or Resume Anticoagulation After ICH
There is some information available on which to base decisions regarding starting an anticoagulant or antiplatelet agent in a patient with ICH related to oral anticoagulants. Most of these patients have anticoagulant-associated ICH, because it is in these cases that the question of whether to resume treatment usually arises. These patients are most commonly taking anticoagulants for atrial fibrillation and mechanical heart valves. The only other common situation is when a patient with ICH develops a deep vein thrombosis (DVT) or pulmonary embolism.
There are very few data on the risk of recurrent ICH when a patient with anticoagulant-associated ICH is restarted on anticoagulants. There are studies on the baseline risk of recurrent ICH. Follow-up of 243 patients who were able to return home after a first ICH and were followed for a mean of 5.5 years found the risk of recurrent ICH after a first hemorrhage was 1% in the first 3 months.44 The risk of recurrence was 2.1% per year, and higher with increasing age and male sex. If the patient was treated with anticoagulants, which usually was done for prosthetic heart valves, atrial fibrillation, or arterial occlusive disease in this study, the risk of recurrent ICH tripled. A systematic review of the literature found that spontaneous, primary lobar hemorrhages, which are often seen in the elderly and are attributed to amyloid angiopathy, have a higher risk of recurrence, probably 4% per year.45
Claassen et al46 followed 55 patients with anticoagulant-associated ICH. Anticoagulation was restarted 7 to 28 days after ICH in 23 patients, 10 of whom had mechanical heart valves. Mortality during follow-up was not significantly different from that in patients in whom anticoagulation was not restarted. After a mean follow-up of 43 months, there were three fatal and two nonfatal hemorrhages in the restarted group, compared with no hemorrhages and one fatal and four nonfatal thromboembolic events. Other studies are small case series included in systematic reviews.47,48 Claassen and colleagues concluded that anticoagulation could be resumed in some cases and the clinician should consider the patient’s risk of falls, general medical condition, and risk factors for systemic hemorrhage.
American Heart Association guidelines on patients with heart valves have not addressed the issue of resuming anticoagulation after ICH.49,50 There are at least three systematic reviews addressing whether to resume anticoagulation in patients with anticoagulant-associated ICH.47,48,51 One review found seven publications (one epidemiologic study and six case series) describing 42 patients treated with anticoagulants for at least 6 months after ICH.46,48 These patients had four recurrent ICHs and nine thromboembolic events. The authors concluded that there was insufficient data upon which to base any decisions. Balancing the risk of thromboembolic events without treatment, for which there are data, with the risk of recurrent ICH after restarting anticoagulants is impossible because there are no data on the latter. The second systematic review included more studies and numerous case reports in addition to the case series because it included all types of intracranial hemorrhage and investigated whether it was safe to resume anticoagulation in patients with mechanical heart valves.47 But the overall quality of the data was judged to be poor. Among case series, anticoagulants were stopped for 2 days to 3 months. During a mean follow-up of 8 months, there were four ischemic strokes and two hemorrhages (one fatal). Among 18 case reports, there were two hemorrhages (one fatal) and no ischemic strokes. The authors concluded that stopping anticoagulation for a few days (and even 7 to 14 days) was safe and that resuming anticoagulation also was safe. According to commonly used evidence-based medicine guidelines, these conclusions would be very weak, but resuming anticoagulation in patients with mechanical heart valves does seem to be common practice. Another consideration is that the risk of stroke and thromboembolic events in patients with mechanical heart valves varies depending on the location and type of valve.52 The risk is higher for mechanical rather than tissue valves, higher for mitral than aortic valves, and higher if there is associated atrial fibrillation (Table 19.3). Overall, the risk of valve thrombosis and major or minor embolic events is 9 to 22% per year.47
Table 19.3 Estimates for Risk of Thromboembolic Events for Various Cardiac Conditions52
| Annual Risk of Thromboembolic Events (%) |
Bioprosthetic aortic valve | 0.5–2% |
Bioprosthetic mitral valve | 0.4–4% |
Mechanical aortic valve | 0.3–4% |
Mechanical mitral valve | 0.5–8% |
Intracardiac thrombus | 3–15% |
Long-term anticoagulation is most commonly indicated for nonvalvular atrial fibrillation. In these patients, the risk of ischemic stroke is 2 to 5% per year but is stratified based on several different grading systems. One is the CHADS2 scoring system (Table 19.4).53,54 Risk of ischemic stroke varies from 2% per year in patients with a score of 0, to 18% in those with a score of 6.53 Examining this scale suggests that in patients with prior ICH, it may not be recommended to start or resume anticoagulation for patients with atrial fibrillation and a low CHADS2 score, for example, patients without prior ischemic stroke or transient ischemic attack plus at least another risk factor, or with three or four risk factors, because their risk of ischemic stroke (4% per year) is similar to the risk of hemorrhage without anticoagulants. One also has to consider that the morbidity and mortality from anticoagulant-associated ICH is higher than that from ischemic stroke. The decision to resume anticoagulation in those with more risk factors for stroke would be made on a case-by-case basis.
Table 19.4 CHADS2 Score to Predict Risk of Stroke in Patients with Atrial Fibrillation53
Points | Annual Risk of Stroke (%) |
0 | 1.9 |
1 | 2.8 |
2 | 4.0 |
3 | 5.9 |
4 | 8.5 |
5 | 12.5 |
6 | 18.2 |
Note: Patients with 1 point: congestive heart failure, hypertensive history, age >75 years, diabetes; patients with 2 points: prior ischemic stroke or transient ischemic attack.
Attempts to model this determination with decision analysis have been made, but the data to base the models on, as noted above, are poor or nonexistent. One decision analysis found that anticoagulation after anticoagulation-induced ICH could seldom be recommended, except perhaps in a patient with deep ICH, controlled hypertension, and a high risk of thromboembolic events.55 The indications have to be extremely compelling in patients with lobar ICH or microhemorrhages on MRI, because these patients often have amyloid angiopathy and are at higher risk of recurrent ICH (4 to 15% per year) without anticoagulants.55 These recommendations are consistent with those in American Heart Association guidelines for management of ICH.27 Those guidelines state that patients with nonvalvular atrial fibrillation and lobar ICH should probably not be restarted on anticoagulants (class 2a, level B evidence) and that anticoagulation may be considered after nonlobar ICH when there are definite indications for anticoagulation (class 2b, level B evidence).
It is important to control risk factors—such as hypertension, diabetes, and smoking—for vascular disease in all cases. There should be no indication of recurrent ICH on repeat CT scans. Patients generally should be started on unfractionated heparin without a bolus, or preferably a low molecular weight heparin and then bridged to warfarin. A lower target INR goal of 2 might also be considered.
When to Start or Resume Anticoagulation After ICH
If a decision is made to start or resume anticoagulation, when is it safe to do so after ICH? Wijdicks et al56 retrospectively reviewed 39 patients with mechanical heart valves and ICH, subdural hemorrhage, or subarachnoid hemorrhage. Only eight of 26 actually had increased prothrombin times at the time of ICH. Anticoagulation was stopped for 2 days to 3 months (mean of 8 days), and no patient suffered a thromboembolic complication during this time. One patient with bilateral acute subdural hematomas presented 3 years later with an ICH, but the prothrombin time was normal on that admission. The authors suggested that one could resume anticoagulation 1 to 2 weeks after ICH with or without craniotomy. Another series suggested resuming anticoagulation 3 days after craniotomy for ICH.57
Another study reviewed 141 patients with anticoagulant-associated ICH, subdural hematoma, subarachnoid hemorrhage, or intraventricular hemorrhage, and an estimated high risk of thromboembolic events due to mechanical heart valves, atrial fibrillation, recurrent stroke, or transient ischemic attack.58 Anticoagulation was stopped for 0 to 30 days (median of 10 days). The risk of thromboembolic events was 3 to 5% within the first 7 days in all groups. The authors recommended stopping anticoagulation for 1 to 2 weeks before resuming it, if the decision was made to resume it. Tinker and Tarhan59 stopped anticoagulation for a mean of 7 days in 180 patients with mechanical heart valves who were undergoing various surgeries.59 There were no thromboembolic complications.
Numerous studies show that in general anticoagulation can be stopped for days with a low risk of thromboembolic events.60 These reports include patients taking anticoagulants for varying indications who develop different types of intracranial hemorrhages, although the most common indications are for atrial fibrillation and heart valves. One series of 108 patients with ICH or subdural hemorrhage while on anticoagulants found that the risk of thromboembolic events while anticoagulants were stopped was 0.66 events/1,000 patient days.60 Seven of eight recurrent hemorrhages were before anticoagulants were resumed, and the one hemorrhage after starting anticoagulants was after 4 months in a patient with a chronic subdural hematoma.
Another review that focused on resuming anticoagulation after neurosurgery classified patients as high, medium, or low risk of recurrent thromboembolic events.52 High-risk patients included those with high-risk peripheral vascular grafts within 9 months of bypass, recent arterial thromboembolic events, or intracardiac thrombi. These authors stopped anticoagulants for intracranial surgery, treated the patient with subcutaneous low-dose heparin postoperatively, and resumed anticoagulation with heparin (no bolus) followed by warfarin 3 to 5 days after surgery. Moderate-risk patients included mainly those with mechanical mitral or combined valves, valvular atrial fibrillation, atrial fibrillation with a recent thromboembolic event, and patients with older peripheral vascular grafts. These patients were treated with low-dose subcutaneous heparin postoperatively and then started on anticoagulation 5 to 7 days after surgery. In the low-risk group of patients with more chronic atrial fibrillation, aortic valve replacements, bioprosthetic mitral valves, and such, again low-dose subcutaneous heparin was administered postoperatively and anticoagulation resumed 7 to 14 days after surgery.
A systematic review found several case series and case reports in which anticoagulants were stopped for 2 days to 3 months after various types of intracranial hemorrhage.48 Most physicians stopped anticoagulants for 7 to 14 days.
A third systematic review included all types of intracranial hemorrhage.51 The authors noted that most recurrent intracranial hemorrhage occurred within 3 days of the first hemorrhage, and most thromboembolic events occurred later than 3 days.51 Only 13% of patients were on anticoagulants when they rebled, and patients with subdural hematoma were more likely to rebleed. The authors concluded that it might be reasonable to resume anticoagulation, if indicated, more than 3 days but earlier than 7 days after ICH, which is sooner than generally done.
For patients with mechanical heart valves, the overall risk of thromboembolic events was in the worst case scenario estimated to be 10 to 20% per year.49,50 In the American Heart Association guidelines, it is generally recommended to stop anticoagulation for 3 days in patients with mechanical valves who are undergoing surgery. The risk of a thromboembolic event during this time would be 0.08 to 0.16%. The guidelines do not address intracranial surgery or patients with ICH.
Venous Thromboembolism Prevention and Treatment for Patients with Intracerebral Hemorrhage
Rarely, a patient with an unruptured aneurysm or known brain vascular malformation develops venous thromboembolic disease. More commonly, patients admitted with ICH develop venous thromboemboli and are candidates for anticoagulation.
The first issue to decide is the use of prophylactic treatment in these patients. The American College of Chest Physicians’ 2008 anticoagulation guidelines give evidence-based practice guidance on venous thromboembolism prophylaxis for patients with ICH.61 The guidelines recommend the initial use of intermittent pneumatic devices (grade 1B evidence) and that in stable patients, with no indication of hematoma growth, low-dose subcutaneous heparin can be safely started as early as the second day after hemorrhage (grade 2C evidence). American Heart Association guidelines on the management of ICH reported that intermittent pneumatic compression combined with elastic stockings was superior to elastic stockings alone in reducing occurrence of asymptomatic deep vein thrombosis after ICH in one randomized trial (4.7% versus 15.9%).27,62 Graduated compression stockings by themselves were of no use.63 Whether to add pharmacological prophylaxis to mechanical compression methods has been studied in patients with ICH, but the studies are small and did not find any differences in the incidence of deep vein thrombosis or bleeding.64,65 The guidelines concluded that after ICH, patients should have intermittent pneumatic compression plus elastic stockings (class 1, level of evidence B). The recommendation for starting pharmacological prophylaxis was the same as other guidelines, in that once there was no evidence of ongoing intracranial bleeding, low-dose subcutaneous low molecular weight or unfractionated heparin could be considered in patients with ICH who were immobile, beginning 1 to 4 days after the ICH (class 2b, level of evidence B).27
In neurosurgery patients undergoing craniotomy, a meta-analysis was conducted of eight randomized clinical trials comparing low-dose unfractionated or low molecular weight heparin with controls for prevention of venous thromboembolism after surgery.66 Although the risk of venous thromboembolism was nearly halved with pharmacological prophylaxis, the risk of hemorrhage was increased as well, making decision making complex. In patients with unruptured aneurysms and vascular malformations who require short-term perioperative pharmacological prophylaxis, the benefits would generally outweigh the risk of hemorrhage.
There is lack of available evidence regarding optimal treatment of venous thromboembolism in patients with acute ICH. We, therefore, rely on the following observations. In patients with acute ICH, the risk of fatal pulmonary embolus is 25% for those with an untreated proximal deep vein thrombosis. On the other hand, the risk of recurrent ICH while on anticoagulants is 3 to 5% (three- to fivefold risk over placebo).61 Hence, treatment with anticoagulants is justified as the risk of fatal pulmonary embolism is high. Unfractionated heparin and low molecular weight heparins are highly efficacious and could be started days after ICH, and then the patient bridged to reduced doses of oral anticoagulation (with a target INR of 2.0) for 3 to 6 months. Pharmacological prophylaxis could be continued with serial imaging to rule out extension in patients with deep vein thrombosis below the knee. Placement of vena cava filters is a reasonable option in patients judged to be at high risk of rebleeding after ICH, intracranial surgery, or rupture of a vascular lesion.
Antiplatelet Drugs and ICH
Data are conflicting on whether patients taking antiplatelet drugs have a higher risk of primary ICH, or have a poorer outcome if they have ICH compared with those not taking these drugs.67,68 Measurement of platelet function showed a poor correlation between the reported use of antiplatelet drugs and platelet function in patients with ICH, suggesting that the history may be unreliable and that this could explain the difficulty in showing correlations among antiplatelet use, ICH expansion, and outcome.67 Analyses of randomized clinical trials found minimal or no effect of prior antiplatelet use on ICH expansion after hospital admission.68 The utility of platelet transfusion in patients admitted with an ICH associated with antiplatelet use was considered investigational in recent ICH guidelines.27 Exploratory observations on use of acetylsalicylic acid from the International Stroke Trial and Chinese Acute Stroke Trial found 773 patients were randomized to aspirin or placebo before they had a CT scan showing that they had an ICH rather than an ischemic stroke.69 This was not associated with an increased risk of death. These data are not very useful when considering whether to start antiplatelet drugs after ICH because they are not population-based, and patients on antiplatelet drugs who die or are not treated are not entered into these studies, which could underestimate the risks.
There is one study addressing the use of antiplatelet therapy in patients with prior ICH48,70; 207 patients who survived an ICH were followed for a median of 20 months, 46 of whom (22%) were treated with antiplatelet drugs. There were 32 recurrent ICHs (20%) among 161 patients who did not receive antiplatelet drugs and seven ICHs (15%) among the 46 who did. Ischemic cardiovascular events occurred in seven untreated and four treated patients. Overall, there were no significant differences in risk of recurrent lobar ICH in patients on antiplatelet drugs (hazard ratio [HR], 1.2; 95% CI, 0.4–3.3) or risk of deep ICH (HR, 1.2; 95% CI, 0.1–14.3) or ischemic events (HR, 1.2; 95% CI, 0.3–4.8). Experts have conflicting opinions about these data. Some note that the confidence intervals are wide, which suggests that antiplatelet drugs should only be administered after ICH in patients at high risk of ischemic events, and that more data are needed.71 Others consider this study more definitive, that antiplatelet drugs do not increase the risk of recurrent ICH and that they should be administered if otherwise indicated.72
One approach is to resume or start acetylsalicylic acid at a low dose (81 mg/d) in patients with strong indications, such as clinically important coronary artery disease or atrial fibrillation.55 The indications have to be increasingly compelling in patients with lobar ICH or microhemorrhages on MRI, because these patients often have amyloid angiopathy and are at higher risk of recurrent ICH (4 to 15% per year) without antiplatelet drugs.55 The indication for antiplatelet drugs could be weaker in patients with deep ICH, where the risk of recurrence is lower (2% per year).55 It is important to control risk factors for vascular disease in all cases, such as hypertension, diabetes, and smoking. The American Heart Association guidelines on management of ICH state that antiplatelet therapy after lobar or deep ICH can be considered when there are definite indications for these drugs (class 2b, level of evidence B).27
KEY POINTS
• The decision regarding the use of anticoagulant and antiplatelet agents in patients with concomitant neurovascular conditions is made in the following way: the best available estimate of the natural risk of hemorrhage in that condition is weighed against the best estimate of the risk of the event that the agent is meant to prevent. Added to that, as always in neurosurgery, is the consideration of the lesion location and the possibility of catastrophic consequences from worsening the severity of any hemorrhage in that location.
• A prolonged prothrombin time can be caused by warfarin, vitamin K deficiency, liver dysfunction, congenital factor VII deficiency, or mild DIC.
• A prolonged activated partial thromboplastin time can be caused by heparin, lupus anticoagulant, deficiencies of factors VIII, IX, or XI, and von Willebrand disease.
• A prolonged thrombin time can be caused by fibrinogen abnormalities and inhibitors of the conversion of fibrinogen to fibrin, the final step of the coagulation cascade.
• The best available evidence supports the conclusion that aspirin does not increase the risk of rupture in patients with unruptured aneurysms. Patients with known aneurysms, however, should be counseled to avoid antiplatelet agents unless it is likely that their risk of stroke, vascular, or cardiac event is higher than their estimated risk of aneurysm rupture.
• In general, patients with arteriovenous malformations (AVMs) should avoid taking antiplatelet and anticoagulant medications. The exceptions would include patients with untreatable AVMs who have a high risk of morbidity from stroke, cardiac ischemic disease, or other vascular disease, or of death.
• Patients with cavernous malformations (CMs) should be counseled to avoid antiplatelet and anticoagulant medications unless there is a compelling medical indication for their use. As opposed to AVMs and aneurysms, however, the low risk of hemorrhage and the generally low mortality of hemorrhage from CMs will figure importantly in the risk/benefit analysis.
• Patients on anticoagulants who present with aneurysm rupture or symptomatic hemorrhage from an AVM or cavernous malformation should have their anticoagulation reversed along the guidelines used for anticoagulation-associated intracerebral hemorrhage (ICH).
• The decision to restart anticoagulation after ICH remains a difficult one because of the lack of quality evidence. Patients with nonvalvular atrial fibrillation or patients with lobar ICH should probably not be restarted on anticoagulants because of the high risk of recurrent ICH. Patients with nonlobar ICH and strong indications for anticoagulation should be considered candidates for restarting anticoagulation. The timing of restarting anticoagulation remains controversial, and should be based on the perceived risk of a thromboembolic event.
• Resumption of antiplatelet drugs in patients with ICH can be considered in those at low risk for recurrent ICH, specifically nonlobar hemorrhage and no evidence on MRI of microhemorrhages associated with amyloid angiopathy.
• Prophylaxis of venous thromboembolic events in patients with ICH is an emerging field of study. Many centers begin pharmacological prophylaxis with low molecular weight or unfractionated heparin 1 to 4 days after ICH, provided there is no evidence of ongoing hemorrhage. Inferior vena caval filters remain a reasonable option in patients judged to be at high risk of recurrent hemorrhage or after rupture of a vascular lesion.